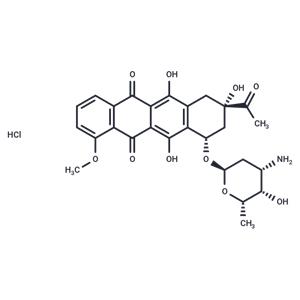
| Name | Daunorubicin hydrochloride |
| Description | Daunorubicin hydrochloride (Rubidomycin hydrochloride), an anthracycline aminoglycoside antineoplastic, inhibits DNA replication and repair and RNA and protein synthesis. |
| Cell Research | Daunorubicin (Dnr) is prepared in PBS[2]. The chemosensitivity to Daunorubicin is assessed using the MTT assay. In brief, the 96 well plates are set up with cells at the initial density of 2×105 cells/mL and are incubated at 37°C for 72 h in an atmosphere of 5% CO2 in the absence and presence of nine different concentrations of Daunorubicin (Dnr) or Dox ranging from 1.90 to 0.007 μM in triplicate. After incubation, 10 μL of MTT solution (5 mg/mL tetrazolium salt) is added to each well and the plates are incubated for a further 4 h at 37°C. The formazan salt crystals are dissolved by adding 100 μL 10% SDS in 10 mM HCl solution and incubating over night at 37°C. The absorbance is measured at 540 nm with a reference at 650 nm by a 96-well enzyme-linked immunosorbent assay (ELISA) plate reader. Chemosensitivity is expressed as the IC50, which is the concentration of drug causing 50% cell survival compare to control cells grown without drug. Calculations are carried out using Microsoft Excel[2]. |
| In vitro | At drug concentrations that reflect the peak plasma concentration after Daunorubicin administration, the primary mechanism is likely to be through interaction with topoisomerase II, which may be a primary triggering event for growth arrest and/or cell killing through a signalling pathway leading to apoptosis, at least in leukemic cells and thymocytes. The quinone structure permits daunorubicin to act as electron acceptors in reactions mediated by oxoreductive enzymes including cytochrome P450 reductase, NADH dehydrogenase, and xanthine oxidase. At Daunorubicin concentrations exceeding approximately 2–4 μM, free radical mediated toxicity and DNA cross-linking may become evident. Daunorubicin inhibits both DNA and RNA syntheses in HeLa cells over a concentration range of 0.2 through 2 μM. Daunorubicin inhibits both DNA syntheses in Ehrlich ascites tumor cells over a concentration range of 4 μM. Daunorubic triggers apoptosis at concentrations of 0.5 and 1 μM in either HL-60 or U-937 human leukemic cells. [1] Daunorubicin stimulates ceramide elevation and apoptosis in P388 and U937 cells through de novo synthesis via activation of the enzyme ceramide synthase. [2] Daunorubicin dose-dependently increases the phosphatidylserine exposure and consequent procoagulant activity of human umbilical vein endothelial cells. Daunorubicin (0.2 mM) significantly enhances the release of endothelial microparticles which are highly procoagulant in human umbilical vein endothelial cells. [3] |
| In vivo | Urinary protein excretion, serum creatinine, and blood urea nitrogen (BUN) level are significantly increased in group Daunorubicin (3 mg/kg, i.v.) compared with those in group Control. Administration of Daunorubicin (DNR) causes a significant increase in malondialdehyde (MDA) level in renal tissue compared with that in the control group[5]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | H2O : 92 mg/mL (163.12 mM), Sonication is recommended.
DMSO : 252.5 mg/mL (447.7 mM), Sonication is recommended.
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 5 mg/mL (8.87 mM), Sonication is recommended.
Ethanol : < 1 mg/mL (insoluble or slightly soluble)
|
| Keywords | Topoisomerase | Rubidomycin | RP13057 Hydrochloride | RP-13057 | RP13057 | RP 13057 Hydrochloride | RP 13057 | RNASynthesis | RNA Synthesis | pancreatic carcinoma leukemia | Molt-4 cell | L3.6 cell | K562 cells | Ehrlich ascites tumor | DNASynthesis | DNA/RNA Synthesis | DNA Synthesis | DNA synthesis | Daunorubicin | cytotoxin | chamomile | Bacterial | Autophagy | Apoptosis | Antibiotic | ADCCytotoxin | ADC Cytotoxin |
| Inhibitors Related | Neomycin sulfate | Cysteamine hydrochloride | Kanamycin sulfate | Sulfamethoxazole sodium | Terbinafine hydrochloride | Hydroxychloroquine | Doxycycline | Tributyrin | Paeonol | Thymidine | Dimethyl sulfoxide | Alginic acid |
| Related Compound Libraries | Anti-Tumor Natural Product Library | Polyphenolic Natural Product Library | Failed Clinical Trials Compound Library | EMA Approved Drug Library | Anti-Cancer Clinical Compound Library | Natural Product Library | Anti-Cancer Approved Drug Library | Natural Product Library for HTS | Anti-Aging Compound Library | Anti-infective Natural Product Library | Anti-Cancer Drug Library | Anti-Cancer Active Compound Library |

 United States
United States