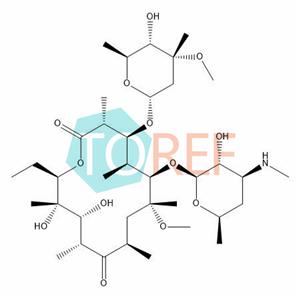
Clarithromycin EP Impurity D
CAS number: 101666-68-6
Product Code: REF-C22004
Molecular formula:C37H67NO13
Molecular weight: 733.94
Purity: 97.29%
Product nature: Customized for customers
Appearance characteristics: White Powder
Storage conditions: -20 ℃
Chemical name: (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-14-ethyl-12,13-dihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-6-[(2S,3R,4S,6R)-3-hydroxy-6-methyl-4-(methylamino)oxan-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione
Hot Tags:Clarithromycin EP Impurity D, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock。
Synthesis of Clarithromycin EP Impurity D
Clarithromycin EP Impurity D Reference Standards,
Complex Impurity Of Clarithromycin EP Impurity D
Chemical Standards of Clarithromycin EP Impurity D
Characterization Of Unknown Impurities of Clarithromycin EP Impurity D
Structure Profiling of Clarithromycin EP Impurity D
Identification of Clarithromycin EP Impurity D
Isolation & Purification of Impurity of Clarithromycin EP Impurity D
Difficult of Clarithromycin EP Impurity D
Instability of Clarithromycin EP Impurity D
Low-assay of Clarithromycin EP Impurity D
Complex Impurity Of Chemical Standards of Clarithromycin EP Impurity D
TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.




 China
China