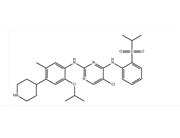
Seretinib property Melting point 173-175 ° C Boiling point 720.7 ± 70.0 ° C (Predicted) Density 1.251 ± 0.06g/cm3 (Predicted) Storage condition -20 ° CFreezer solubility Chloroform (Slightly), Methanol (Slightly) Acid dissociation constant (pKa) 10.16 ± 0.10 (Predicted) Form Off whitesolid. Color WhitetoOff White Use and synthesis of Seretinib for treatment of non-small cell lung cancer (NSCLC) Lung cancer is one of the main causes of cancer death. Non small cell lung cancer (NSCLC) accounts for 85% -90% of lung cancer cases, with 2% -7% of cases driven by rearrangement of the ALK gene, leading to accelerated growth of cancer cells. Although significant progress has been made in clinical treatment of the ALK+NSCLC population, worsening of the condition is often inevitable, requiring more treatment options. Seretinib (LDK378) is a new ALK inhibitor (ALKi) developed by Novartis Pharmaceuticals under the trade name Zykadia. It was approved for sale by the FDA on April 29, 2014, with the code LDK378. It is used to treat non small cell lung cancer (NSCLC) with anaplastic lymphoma kinase (ALK) positive metastasis and progression or intolerance to clotzolitinib. Whether it is detecting enzyme reactions, cell analysis, or animal models of clotozantinib (CRZ) resistance, the results show that it is more effective than CRZ. On September 27, 2013, Seretinib was granted the status of Orphan drug for this indication. Earlier, NEJM reported that out of 80 patients who had previously received clotozantinib treatment, the overall effective rate was 56%. Researchers also observed that ceritinib seretinib was effective regardless of whether there were ALK resistant mutations. Among NSCLC patients who received at least 400mg ceritinib treatment daily, the median progression free survival was 7 months. Among NSCLC patients with advanced ALK rearrangement, including those who have progressed to disease after receiving treatment with clotozantinib, Ceritinib seretinib is highly effective regardless of whether ALK resistance mutations occur. Both medication compliance and existing efficacy results will have a significant impact on Pfizer's new star drug, crisotinib.

 China
China