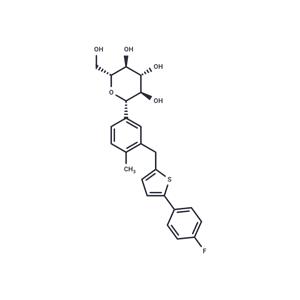
| Name | Canagliflozin |
| Description | Canagliflozin (JNJ 28431754AAA) is a selective SGLT2 inhibitor that inhibits CHO cells expressing mSGLT2, rSGLT2, and hSGLT2 (IC50=2/3.7/4.4 nM). Canagliflozin can be used for the treatment of type II diabetes mellitus (T2DM). |
| Cell Research | Cells from the rat skeletal muscle cell line, L6, is used to test the effect of Canagliflozin on glucose transporter 1 (GLUT1) activity. Cells are maintained in Dulbecco's modified Eagle's medium containing 5.6 mM glucose supplemented with 10% fetal bovine serum, are seeded in 24-well plates at a density of 3 × 105 cells/well and cultured for 24 hours in an atmosphere of 5% CO2 at 37 °C. Cells are rinsed twice with Kreb's ringer phosphate HEPES buffer (pH 7.4, 150 mM NaCl, 5 mM KCl, 1.25 mM MgSO4, 1.25 mM CaCl2, 2.9 mM Na2HPO4, 10 mM HEPES) and are pre-incubated with the solutions of Canagliflozin (250 μL, 10 μM) for 5 minutes at room temperature. The transport reaction is initiated by adding 50 μL of 4.5 mM 2-DG (a substrate for GLUTs)/3H-2-DG (0.625 μCi) followed by incubation for 15 minutes at room temperature. The 2-DG uptake is halted by aspiration of the incubation mixture. Cells are immediately washed 3 times with ice-cold PBS. Samples are extracted with 0.3 N NaOH, and radioactivity is determined by liquid scintillation.(Only for Reference) |
| In vitro | METHODS: CHOK-hSGLT1 and CHOK-hSGLT2 cells were incubated with Canagliflozin (0.01-10000 nM) for 10 min and sodium-dependent glucose uptake was detected.
RESULTS: Canagliflozin inhibited 14C-AMG uptake in CHO-hSGLT1 and CHOK-hSGLT2 cells with IC50 of 684±159 nM and 4.4±1.2 nM, respectively.[1]
METHODS: Human umbilical vein endothelial cell HUVECs were treated with Canagliflozin (1-50 µM) for 24 h and DNA synthesis was measured to monitor cell proliferation.
RESULTS: Treatment of HUVECs with Canagliflozin resulted in a concentration-dependent inhibition of DNA synthesis. The ability of Canagliflozin to block DNA synthesis was observed at concentrations (5-10 µM) achieved in the plasma of patients receiving Canagliflozin. In addition, higher concentrations of Canagliflozin (50 µM) virtually eliminated DNA synthesis. [2] |
| In vivo | METHODS: To study the efficacy of treatment of type 2 diabetes mellitus (T2DM), Canagliflozin (0.1-30 mg/kg, 0.5% hydroxypropyl methylcellulose) was administered by gavage to four animal models. The animal models included: (1) male C57BL/6J mice fed a high-fat diet; (2) male C57BL/ksj-db/db hyperglycemic mice; (3) male Zucker fatty (ZF) obese, insulin-resistant rats; and (4) male ZDF obese, hyperglycemic rats.
RESULTS: Canagliflozin decreased RTG and increased UGE, improved glycemic control and β-cell function, and reduced body weight gain in a rodent model of T2DM. [1] |
| Storage | keep away from direct sunlight,keep away from moisture,store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | H2O : < 1 mg/mL (insoluble or slightly soluble)
DMSO : 50 mg/mL (112.48 mM)
Ethanol : < 1 mg/mL (insoluble or slightly soluble)
|
| Keywords | Sodium-dependent glucose cotransporters | TA-7284 | JNJ-28431754 | Canagliflozin | Inhibitor | inhibit | JNJ28431754 | TA7284 | SGLT |
| Inhibitors Related | Dapagliflozin ((2S)-1,2-propanediol, hydrate) | Ertugliflozin L-pyroglutamic acid | Dapagliflozin | Ipragliflozin | Phlorizin | Empagliflozin | Ertugliflozin |
| Related Compound Libraries | 经典已知活性库 | 抗癌临床化合物库 | 药物功能重定位化合物库 | 抗癌上市药物库 | 已知活性化合物库 | 抗癌药物库 |

 United States
United States