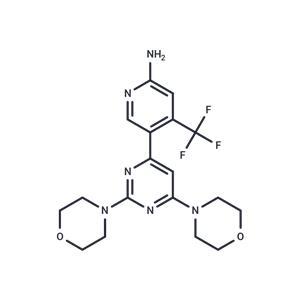
| Name | Buparlisib |
| Description | Buparlisib (BKM120) is an orally bioavailable specific oral inhibitor of the pan-class I PI3K (IC50s: 52/166/116/nM for p110α, p110β, and p110δ). |
| Cell Research | A2780 cells were cultured in DMEM supplemented with 10% FBS. L-glutamine, sodium pyruvate, and antibiotics. Cells were plated in the same medium at a density of 1000 cells per well, 100 ul per well into black-walled-clear-bottom plates and incubated for 3-5 hours. Test compounds supplied in DMSO (20 mM) were diluted further into DMSO (7.5 ul of 20 mM test compound in 22.5 ul DMSO. Mix well, transfer 10 ul to 20 ul DMSO, repeat until 9 concentrations have been made). The diluted test compound solution (2uL), was then added to the cell medium (500 ul) cell medium. Equal volumes of this solution (100 uL) were added to the cells in 96 well plates and incubated at 37 oC for 3 days and developed using Cell Titer Glo. Inhibition of cell proliferation was determined by luminescence read using Trilux [1]. |
| Kinase Assay | Compounds to be tested were dissolved in DMSO and directly distributed into a black 384-well plate at 1.25 μL per well. To start the reaction, 25 μL of 10 nM PI3 kinase and 5 μg/mL 1-alpha-phosphatidylinositol (PI) in assay buffer (10 mM Tris pH 7.5, 5 mM MgCl2, 20 mM NaCl, 1 mM DTT and 0.05% CHAPS) were added into each well followed by 25 μL of 2 μM ATP in assay buffer. The reaction was performed until approx 50% of the ATP was depleted, and then stopped by the addition of 25 μL of KinaseGlo solution. The stopped reaction was incubated for 5 minutes and the remaining ATP was then detected via luminescence [1]. |
| Animal Research | Six- to eight-week-old female severe combined immunodeficiency (SCID) mice were housed and monitored in the MD Anderson Cancer Center animal research facility. SCID mice were subcutaneously inoculated in the right flank with 1 million ARP-1 or MM.1S cells suspended in 50 μl phosphate-buffered saline (PBS). After palpable tumor developed (tumor diameter ≥5 mm), mice were treated with intraperitoneal injection of DMSO/PBS or BKM120 (5 μM per kg per day) for 15 days. Tumor sizes were measured every 5 days, and blood samples were collected at the same period. Tumor burdens were evaluated by measuring tumor size and detecting the circulating human kappa chain or lambda chain [3]. |
| In vitro | Buparlisib exhibited 50-300 nM activity for class I PI3K's, including the most common p110R mutants. Additionally, 15 exhibited lower potency against class III and class IV PI3K's, where 2, 5, >5, and >25 μM biochemical activity was observed for inhibition of VPS34, mTOR, DNAPK, and PI4K, respectively. Across all cell lines, pathway modulation and antiproliferative activity was consistent with cellular PI3K inhibition [1]. Buparlisib demonstrated anti-proliferative activity in 11 human gastric cancer cell lines by decreasing mTOR downstream signaling. But Buparlisib treatment increased p-AKT by subsequent abrogation of feedback inhibition by stabilizing insulin receptor substrate-1. In KRAS mutant gastric cancer cells, either p-ERK or p-STAT3 was also increased upon treatment of Buparlisib [2]. BKM120 induces cell growth inhibition and apoptosis in both multiple myeloma (MM) cell lines and freshly isolated primary MM cells. In addition, BKM120 shows synergistic cytotoxicity with dexamethasone in dexamethasone-sensitive MM cells. Low doses of BKM120 and dexamethasone, each of which alone has limited cytotoxicity, induce significant cell apoptosis in MM.1S and ARP-1. BKM120 exposure causes cell cycle arrest by upregulating p27 (Kip1) and downregulating cyclin D1 and induces caspase-dependent apoptosis by downregulating antiapoptotic XIAP and upregulating expression of a cytotoxic small isoform of Bim, BimS [3]. |
| In vivo | In A2780 xenograft tumors, oral administration of Buparlisib at 3, 10, 30, 60, and 100 mg/kg resulted in dose-dependent modulation of pAKTSer473. Partial inhibition of pAKTSer473 was observed at 3 and 10 mg/kg, with near complete inhibition at 30, 60, or 100 mg/kg. Inhibition of pAkt corresponded with plasma and tumor drug exposure and was time-dependent, with over 90% target modulation achieved at 60 and 100 mg/kg doses at the 10-hour mark when plasma and tumor exposure was approximately 2 μM [1]. Buparlisib treatment (5 μM/kg/day) significantly reduced tumor burdens and circulating human kappa chain levels, also extending the survival of tumor-bearing mice [3]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 76 mg/mL (185.2 mM)
H2O : Insoluble
Ethanol : 2 mg/mL
|
| Keywords | Phosphoinositide 3-kinase | inhibit | BKM 120 | Buparlisib | Inhibitor | PI3K | NVP-BKM-120 | BKM-120 | Apoptosis | NVP-BKM 120 |
| Inhibitors Related | Stavudine | 5-Fluorouracil | Myricetin | Sodium 4-phenylbutyrate | L-Ascorbic acid | Dextran sulfate sodium salt (MW 4500-5500) | Metronidazole | Sorafenib | Tributyrin |
| Related Compound Libraries | Bioactive Compound Library | Kinase Inhibitor Library | Hematonosis Compound Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Anti-Cancer Active Compound Library | Anti-Cancer Drug Library |

 United States
United States