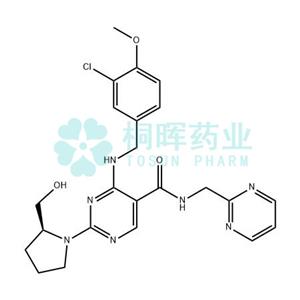
| Name | Avanafil |
|---|
| Cas Number | 330784-47-9 |
|---|
| MF | C23H26ClN7O3 |
|---|
| MW | 483.95 |
|---|
| MDL No. | MFCD11977961 |
|---|
Properties
Melting point:150-152°C
Density 1.372
storage temp. Sealed in dry,Store in freezer, under -20°C
solubility DMSO (Slightly), Methanol (Slightly, Heated)
form Solid
pka11.84±0.46(Predicted)
color White to Off-White
Avanafil API pharmaceutical export
Avanafil API pharmaceutical wholesaler
Shortage Avanafil API Supplies
supply of various Avanafil API
Avanafil API Import and Export Wholesaler
wholesale distribution of Avanafil API
Avanafil API DISTRIBUTION CHANNELS
Wholesaler Distributor Licensure of Avanafil API
Certificate of Analysis (COA) for Avanafil API
Valid Avanafil API GMP certification
Valid Avanafil API Manufacturing License
Method of Analysis for Avanafil API
Material safety data sheet for Avanafil API
Accelerated stability data for Avanafil API
Real time stability data for Avanafil API
Avanafil API Exporters and Contract manufacturing
Avanafil in USP, BP, EP with DMF, Tech Pack, GMP, Written Confirmation
Regulatory Documents of Avanafil
Avanafil API of manufacturers who are USFDA audited & accredited with GMP, ISO
Avanafil with DMF/GMP/ISO (all technical documents support)
Avanafil API in USP/BP/EP pharmacopoeia
Avanafil Micronised, Injection, & all grades
Avanafil API factory accredited by USFDA, Health Canada, TGA, UK-MHRA, PIC/S
Avanafil API with cGMP manufacturing facility、GMP certified facility
Avanafil API with GLP certified laboratory
Avanafil API with cGMP & WHO GMP compliant facility
Avanafil API with R&D and Commercial quantity
Dosage form of Avanafil API
Avanafil API U.sPharmacopeia (UsP), in-house specification and/or European Pharmacopoeia (Ph. Eur)
Avanafil API distributor with a strong supply chain channel
Avanafil API Provide CEP/COS(certificate of suitability to monograph of European Pharmacopoeia) , EDMF(European Drug Master File),and GMP(Good Manufacturing Practice ),Written Confirmation.
Avanafil API certificate of analysis
Avanafil API factory GMP (Goods Manufacturing Practices Certificate )
Avanafil API Product DML (Drug Manufacturing License)
Developed by Japan's Tanabe Pharmaceutical Company and VIVUS, it was approved for listing in the United States on April 27, 2012. This product is a type 5 phosphodiesterase (PDE5) inhibitor, which can increase the blood flow of the penis. It is currently the first-line drug for the treatment of ED. The first-line drug for the treatment of ED includes sildenafil and vardenafil ), tadalafil (tadalafil) and avanafil (avanafil). As a second-generation PDE5 inhibitor, avanafil has the characteristics of fast onset and short duration, and increases the selectivity of PDE5 isoenzymes, which plays an important role in reducing the incidence of adverse reactions. Studies have shown that the drug can take effect in just 15 minutes, which is faster than 30-60 minutes reported by other drugs, and eating can affect the pharmacokinetics of sildenafil and vardenafil, but does not affect alva That's not. The rapid onset of avanafil allows ED patients to enjoy sex freely without having to take medication too early. It is a strong competitor of sildenafil (Viagra).
Hot Tags: Avanafil API, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Tippyridine Hydrochloride API, Lercanidipine Hydrochloride API, Temozolomide API, Dimethyl Fumarate API, Rasagiline Mesylate API, Pleasant API

 China
China