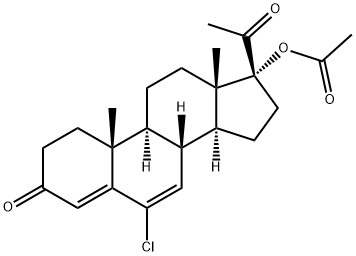
Chemical Properties
Crystalline Solid
Uses
Orally active progesteron with antiandrogenic activity; has been used in combinations as an oral contraceptive. Progestogen; antineoplastic (hormonal)
Uses
tranquilizer, neuroleptic, alpha adrenergic blocker
Brand name
Gestafortin;Luteran;Ovosiston.
World Health Organization (WHO)
Chlormadinone acetate, a synthetic progestogen, was introduced in 1965 as a component in oral contraceptive preparations. In 1967, as a result of new regulations required by the United States Food and Drug Administration, chlormadinone acetate was submitted to long-term toxicity studies and by the early 1970s it was shown to be associated with an increased incidence of mammary tumours in beagle bitches which led to its withdrawal by several regulatory authorities. Subsequently the validity of the beagle bitch model as a predictor of carcinogenicity of steroid contraceptives has been contested by many national regulatory authorities and chlormadinone remains available in some countries for contraceptive purposes. In some instances it is indicated for treatment of progesterone deficiency and endometriosis, and of irregular uterine bleeding due to fibroids. (Reference: (WHODI) WHO Drug Information, 84.1, 5, 1984)
Safety Profile
Suspected carcinogen with experimental carcinogenic and tumorigenic data. Moderately toxic by intraperitoneal route. Human maternal and reproductive effects by ingestion, intramuscular, and possibly other routes: ovary, uterus, cervix, vagina, and fallopian tube changes; menstrual cycle changes or disorders; changes in ferthty; and other unspecified female effects. A human teratogen that causes developmental abnormalities of the endocrine system in the fetus. Experimental teratogenic and reproductive effects. An oral contraceptive. When heated to decomposition it emits toxic fumes of Cl-.

 China
China