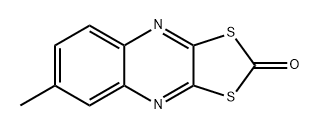
Chemical Properties
Tan to yellow crystals. Solu- ble in benzene, toluene, and dioxane; insoluble in water.
Uses
Acaricide; fungicide.
Uses
Acaricide; agricultural fungicide.
Uses
Chinomethionat exhibits both acaricidal and fungicidal activities. It is a selective, non-systemic contact fungicide with both protective and eradicant control of powdery mildews on fruits, cucurbits, vegetables, ornamentals, tobacco and glasshouse crops.
Definition
ChEBI: A dithioloquinoxaline that results from the formal condensation of 6-methylquinoxaline-2,3-dithiol with phosgene. It has been used as a fungicide and acaricide for the control of mites and powdery mildew on citrus, vegetables, and walnuts, but is not appro ed for use in the EU.
General Description
Yellow crystals. Non-corrosive. Used as a selective fungicide.
Air & Water Reactions
Hydrolyzed in alkaline solution.
Reactivity Profile
A member of the quinoxaline, dithiolane family.
Hazard
Toxic by ingestion and inhalation.
Environmental Fate
Chemical/Physical. Reacts with ammonia forming 6-methyl-2,3-quinoxalinedithiol.
Metabolic pathway
Limited information is available to describe the degradation and metabolic fate of chinomethionat. Hydrolytic cleavage of the dithiocarbonate ring is the primary degradation reaction in water, plants and animals.
Degradation
Chinomethionat was degraded at pH 4,7 and 9 at 22 °C with DT50 values of 10 days, 3.3 days and 3.6 hours, respectively. It is degraded under alkaline conditions to yield 6-methyl-2,3-quinoxalinedithiol (2) (PM) as shown in Scheme 1. Clark and Loeffler (1980) reported the extensive photodegradation of chinomethionat in benzene in a nitrogen atmosphere under UV light to yield dimeric condensation products and benzene insertion products.

 China
China