1/1
Prometryn
$ 1.00
/1KG
- Min. Order1KG
- Purity99%
- Cas No7287-19-6
- Supply Ability100kg
- Update time2019-12-27

career henan chemical co
VIP7Y
 China
China
Since:2014-12-17
Address:702, Building 10, East District, National University Science and Technology Park, High tech Zone, Zh
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Prometryn |
| CAS No | 7287-19-6 |
| EC-No | |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 100kg |
| Release date | 2019/12/27 |
▼
▲
▼
▲
▼
▲
▼
▲
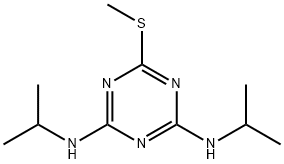
Product Name:
Prometryn
Synonyms:
Prometryn, Gesagard,Caparol,Merkazin,Polisin,Prometrex;CAPAROL;CAPAROL(R);AURORA KA-3878;'LGC' (1627);G34161;GESAGARD;GESAGARD(R)
CAS:
7287-19-6
MF:
C10H19N5S
MW:
241.36
EINECS:
230-711-3
Product Categories:
Alpha sort;Herbicides;N-PAlphabetic;P;Pesticides&Metabolites;PON - PTPesticides&Metabolites;Triazines;NULL;HERBICIDE
Mol File:
7287-19-6.mol
▼
▲
Prometryn Chemical Properties
▼
▲
Melting point
118-120°C
Boiling point
309.64°C (rough estimate)
density
1.157
refractive index
1.5500 (estimate)
Fp
2 °C
storage temp.
APPROX 4°C
form
neat
pka
3.76±0.41(Predicted)
Merck
13,7881
BRN
613575
InChIKey
AAEVYOVXGOFMJO-UHFFFAOYSA-N
CAS DataBase Reference
7287-19-6(CAS DataBase Reference)
NIST Chemistry Reference
1,3,5-Triazine-2,4-diamine, N,N'-bis(1-methylethyl)-6-(methylthio)-(7287-19-6)
EPA Substance Registry System
Prometryn (7287-19-6)
▼
▲
Safety Information
▼
▲
Hazard Codes
F,Xn,N
Risk Statements
11-20/21/22-36-50/53-20
Safety Statements
16-26-36-61-60-36/37
RIDADR
UN 1648 3/PG 2
WGK Germany
2
RTECS
XY4390000
HS Code
29336990
Hazardous Substances Data
7287-19-6(Hazardous Substances Data)
Toxicity
LD50 orally in rats: 3.75 g/kg (Geigy); LC50 (96 hour) in bluegill sunfish, rainbow trout (ppm): 10.0, 2.5 (Tweedy, Kahrs)
▼
▲
MSDS Information
▼
▲
Prometryn Usage And Synthesis
▼
▲
Chemical Properties
Colorless crystalline solid.
Uses
Prometryn is a methylthiotriazine herbicide used for the control of several annual grasses and broadleaf weeds. Prometryn works by inhibiting the electron transport in target broadleaves and grasses.
Uses
Herbicide.
Uses
Selective herbicide used to control many annual grasses and broad-leaved weeds in cotton, celery and peas.
Production Methods
Prometryne is made either by reacting propazine with methyl mercaptan in the presence of one equivalent of NaOH or by reacting 2-mercapto-4,6-bis(isopropylamino)-5-triazine with a methylating agent in the presence of NaOH.
Prometryne is made either by reacting propazine with methyl mercaptan in the presence of one equivalent of NaOH or by reacting 2-mercapto-4,6-bis(isopropylamino)-5-triazine with a methylating agent in the presence of NaOH.
Prometryne is made either by reacting propazine with methyl mercaptan in the presence of one equivalent of NaOH or by reacting 2-mercapto-4,6-bis(isopropylamino)-5-triazine with a methylating agent in the presence of NaOH.
Definition
ChEBI: A diamino-1,3,5-triazine that is N,N'-di(propan-2-yl)-1,3,5-triazine-2,4-diamine substituted by a methylsulfanediyl group at position 6.
General Description
Colorless crystals. Used as an herbicide.
Reactivity Profile
A triazine derivative. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Agricultural Uses
Herbicide: Prometryn is used to control several annual grasses and broadleaf weeds in terrestrial food and feed crops. Its major uses are on cotton and celery, and is often used on dill and pigeon peas. Not approved for use in EU countries. Registered for use in the U.S.
Trade name
A-1114®; CAPAROL®; COTTON PRO®; G 34161®; GESAGARD®; MERCASIN®; MERCAZIN®; MERKAZIN®; POLISIN®; PRIMAPIN®; PRIMATOL-Q®; PROMET®; PROMETREX®; SELECTIN®; SELECTIN-50®; SELEKTIN®; SESAGARD®; SUPREND®; UVON®
Potential Exposure
Prometryn, a triazine herbicide, is used to control several annual grasses and broadleaf weeds in terrestrial food and feed crops. Among its major applica- tions are on cotton and celery and is often used on dill and pigeon peas.
Carcinogenicity
No carcinogenic activity was seen in either the rat or the mouse following lifetime feeding of up to 1500 or 3000 ppm, respectively.
Environmental Fate
Soil. In soil and plants, the methylthio group is oxidized. The proposed degradative pathway is the formation of the corresponding sulfoxide and sulfone derivatives of prometryn followed by oxidation of the latter to forming hydroxypropazine (Kearney and Kaufman, 1976). Cook and Hütter (1982) reported that bacterial cultures degraded prometryne to form the corresponding hydroxy derivative (hydroxyprometryne). 14C-Prometryn was incubated in an organic soil for one year. It was observed that 57.4% of the bound residues were comprised of prometryn (>50%), hydroxypropazine, mono-n-dealkylated prometryn, mono-N-dealkylated hydroxypropazine, a didealkylated compound [2-(methylthio)-4-amino-6-(isopropylamino)-s-triazine] and unidentified methanol soluble products (Khan and Hamilton, 1980; Khan, 1982). Hydroxyprometryn and ammeline were reported as hydrolysis metabolites in soil (Somasundaram et al., 1991). The reported halflife in soil is 60 days (Jury et al., 1987).
Photolytic. When prometryn in aqueous solution was exposed to UV light for 3 hours, the herbicide was completely converted to hydroxypropazine. Irradiation of soil suspensions containing prometryn was found to be more resistant to photodecomposition. About 75% of the applied amount was converted to hydroxypropazine after 72 hours of exposure (Khan, 1982). The UV (λ = 253.7 nm) photolysis of prometryn in water, methanol, ethanol, n-butanol and benzene, yielded 2-methylthio-4,6-bis(isoprop-ylamino)-s-triazine. At wavelengths >300 nm, photodegradation was not observed (Pape and Zabik, 1970). Khan and Gamble (1983) also studied the UV irradiation (λ = 253.7 nm) of prometryn in distilled water and dissolved humic substances. In distilled water, 2-hydroxy-4,6-bis(isopropylamino)-s-triazine and 4,6-bis(isopropylamino)-s-triazine formed as major products.
In the presence of humic/fulvic acids, 4-amino-6-(isopropylamino)-s-triazine was also formed (Khan and Gamble, 1983). Pelizzetti et al. (1990) studied the aqueous photocatalytic degradation of prometryn and other s-triazines (ppb level) using simulated sunlight (λ >340 nm) and titanium dioxide as a photocatalyst. Prometryn rapidly degraded forming cyanuric acid, nitrates, sulfates, the intermediate tentatively identified as 2,4-diamino-6- hydroxy-N,N′-bis(1-methyl-ethyl)-1,3,5-triazine and other intermediate compounds similar to those found for atrazine. It was suggested that the appearance of sulfate ions was due to the attack of the methylthio group at the number two position. Mineralization of cyanuric acid to carbon dioxide was not observed (Pelizzetti et al., 1990).
Chemical/Physical. Mascolo et al. (1995) studied the reaction of prometryn (100 μM) with sodium hypochlorite (10 mM) at 25°C and pH 7. Degradation followed the following pathway: prometryn → 2,4-(N,N′-diisopropyl)diamino-6-methylsulfinyl-1,3,5-triazine → 2,4-(N,N′-diisopropyl)diamino-6-methylsulfonyl-1,3,5-triazine → 2,4-(N,N′-diisopropyl)diamino-6-methanesulfonate ester 1,3,5-triazine → 2,4-(N,N′-diisopropyl)diamino-6- hydroxy-1,3,5-triazine.
Photolytic. When prometryn in aqueous solution was exposed to UV light for 3 hours, the herbicide was completely converted to hydroxypropazine. Irradiation of soil suspensions containing prometryn was found to be more resistant to photodecomposition. About 75% of the applied amount was converted to hydroxypropazine after 72 hours of exposure (Khan, 1982). The UV (λ = 253.7 nm) photolysis of prometryn in water, methanol, ethanol, n-butanol and benzene, yielded 2-methylthio-4,6-bis(isoprop-ylamino)-s-triazine. At wavelengths >300 nm, photodegradation was not observed (Pape and Zabik, 1970). Khan and Gamble (1983) also studied the UV irradiation (λ = 253.7 nm) of prometryn in distilled water and dissolved humic substances. In distilled water, 2-hydroxy-4,6-bis(isopropylamino)-s-triazine and 4,6-bis(isopropylamino)-s-triazine formed as major products.
In the presence of humic/fulvic acids, 4-amino-6-(isopropylamino)-s-triazine was also formed (Khan and Gamble, 1983). Pelizzetti et al. (1990) studied the aqueous photocatalytic degradation of prometryn and other s-triazines (ppb level) using simulated sunlight (λ >340 nm) and titanium dioxide as a photocatalyst. Prometryn rapidly degraded forming cyanuric acid, nitrates, sulfates, the intermediate tentatively identified as 2,4-diamino-6- hydroxy-N,N′-bis(1-methyl-ethyl)-1,3,5-triazine and other intermediate compounds similar to those found for atrazine. It was suggested that the appearance of sulfate ions was due to the attack of the methylthio group at the number two position. Mineralization of cyanuric acid to carbon dioxide was not observed (Pelizzetti et al., 1990).
Chemical/Physical. Mascolo et al. (1995) studied the reaction of prometryn (100 μM) with sodium hypochlorite (10 mM) at 25°C and pH 7. Degradation followed the following pathway: prometryn → 2,4-(N,N′-diisopropyl)diamino-6-methylsulfinyl-1,3,5-triazine → 2,4-(N,N′-diisopropyl)diamino-6-methylsulfonyl-1,3,5-triazine → 2,4-(N,N′-diisopropyl)diamino-6-methanesulfonate ester 1,3,5-triazine → 2,4-(N,N′-diisopropyl)diamino-6- hydroxy-1,3,5-triazine.
Shipping
Triazine pesticides, solid, toxic, n.o.s. require a shipping label of “poisonous materials.” This material fall in DOT/UN Hazard Class 6.1.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recom- mendations for the disposal of pesticides and pesticide containers. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
▼
▲
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;