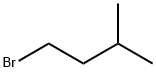
Chemical Properties
clear colourless liquid
Uses
An alkyl halide and organic synthesis reagent.
Uses
In organic synthesis.
General Description
A colorless liquid. Flash point 21°F. Slightly soluble in water and denser than water. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Slightly soluble in water and denser than water.
Reactivity Profile
Halogenated aliphatic compounds, such as 1-Bromo-3-methylbutane, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Low molecular weight haloalkanes are highly flammable and can react with some metals to form dangerous products. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Gives toxic fumes of bromine when burned.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic by intraperitoneal route. Flammable liquid. Dangerous fire hazard when exposed to heat or flame. When heated to decomposition it emits toxic fumes of Br-. See also BROMIDES.
Purification Methods
Shake the bromide with conc H2SO4, wash with water, dry with K2CO3 and fractionally distil it. [Beilstein 1 IV 378.]

 China
China