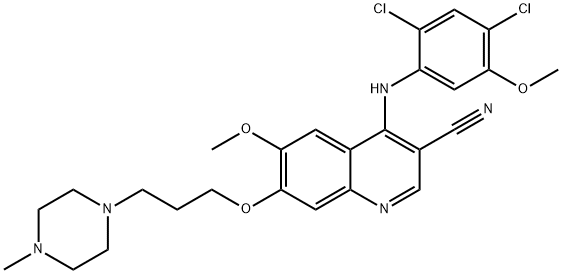
Description
In September 2012, the US FDA approved bosutinib (also referred to as SKI-607) for the treatment of relapsed or refractory chronicmyeloid leukemia (CML) for patients with resistance or intolerance to prior therapy. The National Cancer Institute estimates that in 2012, 5430 men and women were diagnosed with CML and 610 died of CML. First and second-line therapies for the treatment of CML include imatinib, dasatinib, and nilotinib. Bosutinib, which was initially identified as a Src inhibitor, was later found to be a dual inhibitor of Bcr–Abl (IC50=1.4 nM) and Src family kinases (IC50=3.5 nM). Bosutinib inhibits 16 of 18 imatinib-resistant forms of Bcr–Abl expressed in murine myeloid cell lines. In preclinical in vivo studies, bosutinib at 15 mg/kg administered orally for 5 days caused regression of K562 CML tumors in nude mice and in BaF3 tumors expressing wild type or different imatinib-resistant Bcr–Abl mutants at varying doses. A manufacturing process for the synthesis of bosutinib monohydrate has been reported that employs a key three-component cyclization reaction involving an aniline, a cyanoacetamide intermediate, and triethyl orthoformate followed by cyclization using phosphorous oxytrichloride and an optimized hydration procedure.
Chemical Properties
Pale Yellow Solid
Originator
Wyeth (United States)
Uses
Bosutinib (SKI-606) is a novel, dual Src/Abl inhibitor with IC50 of 1.2 nM and 1 nM, respectively.
Uses
A novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells.
Uses
peripheral vasolidator increases cerebral blood flow, potential therapeutic for Altzheimer’s disease, cognitive impairment and dementia
Definition
ChEBI: An aminoquinoline that is 4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline bearing additional cyano and methoxy substituents at positions 3 and 6 respectively.

 China
China