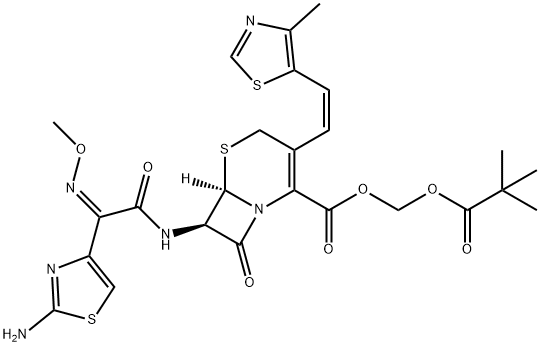
Description
Cefditoren pivoxil is an orally active third generation cephalosporin introduced in Japan as a treatment for a broad range of bacterial infections including dermatological and other community acquired infections. Cefditoren pivoxil is reported to have a broad spectrum of activity against both Gram-positive and Gramnegative bacteria, more potent than many other existing agents of its class. In particular, it shows the highest therapeutic activity against S. pneumoniae and S. marcescens infections. It exhibits resistance to β-lactamase hydrolysis typical of third generation cephalosporins. As a prodrug of cefditoren, it is readily absorbed through GI tract and has low toxicity and side effects.
Chemical Properties
Off-White Powder
Originator
Meiji Seika (Japan)
Uses
An antibacterial. Third generation cephalosporin
Definition
ChEBI: The pivaloyloxymethyl ester prodrug of cefditoren.
Antimicrobial activity
It exhibits good activity against staphylococci, streptococci (but not enterococci), H. influenzae and M. catarrhalis, including β-lactamase-producing strains. Isolates of Str. pneumoniae exhibiting reduced susceptibility to penicillin are less susceptible (MIC 0.125–2 mg/L). Most enterobacteria, including many Enterobacter, Citrobacter, Serratia and Proteus spp., are susceptible. It is not active against Ps. aeruginosa, Sten. maltophilia or atypical respiratory pathogens such as Chlamydophila pneumoniae and M. pneumoniae. It is stable to staphylococcal and common enterobacterial β-lactamases.
Pharmacokinetics
Oral absorption: c. 70%
Cmax 200 mg oral: c. 1.8 mg/L after 1.5–3 h
Plasma half-life: 0.8–1.3 h
Volume of distribution: 9.3 L
Plasma protein binding: 88%
After oral administration the pivaloyl ester is rapidly cleaved by esterases in the gut wall. Ingestion with food improves the bioavailability. Plasma concentrations are raised in elderly patients. There is no accumulation on repeated dosing.
It is excreted unchanged in the urine with a half-life of around 1.5 h, achieving a concentration of 150–200 mg/L within 4 h. Dosage adjustment is recommended in patients with deteriorating renal function.
Clinical Use
It has been advocated for community-acquired upper and lower respiratory tract infections and skin infections.
Side effects
In common with other pivoxil esters it may cause carnitine deficiency. Other side effects are those common to cephalosporins, mainly gastrointestinal disturbance.

 China
China