Description
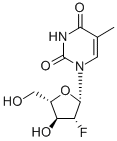
Clevudine is a fluorinated b-L-nucleoside analog launched for the oral treatment of chronic HBV infection. It is the fifth nucleoside or nucleotide analog to be marketed for this indication. The previous drugs from this class include lamivudine, adefovir, entecavir, and telbivudine. In HBV-expressing human hepatoma cell line 2.2.15, clevudine inhibits HBV DNA synthesis with an EC50 of 0.1 μM, and does not show cytotoxicity up to 200 μM. It is phosphorylated by cellular kinases to the active triphosphate derivative, which subsequently inhibits HBV DNA polymerase and HBV replication. 466 Shridhar Hegde and Michelle Schmidt Clevudine-5′-triphosphate has an intracellular half-life of 16.5 h. Interestingly, it is a non-competitive inhibitor of viral polymerase, and inhibits HBV replication without being incorporated into the DNA. The pharmacokinetic profile of clevudine was linear with a plasma half-life of approximately 60 h. Clevudine was undetectable in plasma after 4 weeks following the cessation of dosing.The most common adverse events reported with clevudine treatment include infection, asthenia, dyspepsia, abdominal pain, headache, and diarrhea.
Clevudine is chemically derived from L-ribose by first incorporating acyl protective groups to produce 1-O-acetyl-2,3,5-tri-O-benzoyl-b-L-ribofuranose intermediate, which is then converted to 1,3,5-tri-O-benzoyl-a-L-ribofuranose in two steps by treating To Market, To Market 2007 467 with hydrogen chloride and subsequent hydrolysis and acyl migration. The remaining steps leading to clevudine include conversion of the C2-hydroxy group to C2-fluoro group with triethylamine trihydrofluoride, formation of the corresponding ribofuranosyl bromide intermediate with hydrogen bromide and acetic acid, condensation with silylated thymine, and removal of benzoyl protective groups with methanolic ammonia.

 China
China