Product no. chemwill-GLCAD-9201032
highly quality and immedaite delivery
Gallic acid Manufacturer High quality Best price In stock factory CAS 149-91-7
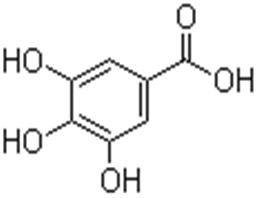
Gallic acid 149-91-7 3,4,5-Trihydroxybenzoic acid
Gallic acid CAS 149-91-7
CAS 149-91-7
CAS 149-91-7
Name: Gallic acid
Synonyms: Gallic acid 149-91-7;TIMTEC-BB SBB008781;RARECHEM AL BE 0070;3,4,5-trihydroxy-benzoicaci;gallic;Gallussaure;Kyselina 3,4,5-trihydroxybenzoova;Kyselina gallova
CAS: 149-91-7
MF: C7H6O5
MW: 170.12
EINECS: 205-749-9
3,4,5-Trihydroxybenzoic acid; Pyrogallol-5-carboxylic acid; Gallic Acid Anhydrous; Gallic acid(anhydrous)
CAS RN.:
149-91-7
EINECS: 205-749-9
Gallic acid is a trihydroxybenzoic acid, a type of phenolic acid, a type of organic acid, also known as 3,4,5-trihydroxybenzoic acid, found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. The chemical formula is C6H2(OH)3COOH. Gallic acid is found both free and as part of hydrolyzable tannins. The gallic acid groups are usually bonded to form dimers such as ellagic acid. Hydrolyzable tannins break down on hydrolysis to give gallic acid and glucose or ellagic acid and glucose, known as gallotannins and ellagitannins respectively.
Gallic acid forms intermolecular esters (depsides) such as digallic and trigallic acid, and cyclic ether-esters (depsidones).
Gallic acid is commonly used in the pharmaceutical industry. It is used as a standard for determining the phenol content of various analytes by the Folin-Ciocalteau assay; results are reported in gallic acid equivalents. Gallic acid can also be used as a starting material in the synthesis of the psychedelic alkaloid mescaline.
The name is derived from oak galls, which were historically used to prepare tannic acid. Despite the name, gallic acid does not contain gallium. Salts and esters of gallic acid are termed "gallates".
Appearance & Physical State white crystalline powder
Density 1.694
Boiling Point 501.1ºC at 760 mmHg
Melting Point 251ºC (dec.)
Flash Point 271ºC
Refractive Index 1.73
Water Solubility 12 g/L cold water
Stability Stability Stable, but may discolour upon exposure to light. Hygroscopic. Incompatible with strong oxidizing agents, strong bases, acid chlorides, acid anhydrides.
Storage Condition Do not store in direct sunlight. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from light.
Vapor Pressure 7.32E-11mmHg at 25°C
Safety Info
RTECS LW7525000
Safety Statements S24/25
HS Code 2918290000
Product Categories: chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Fine chemicals;-;Food additive,Antioxidant anticorrosive;Inhibitors;Pharmaceutical Intermediates;Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts
Mol File: 149-91-7.mol
Gallic acid Structure
Gallic acid Chemical Properties
Melting point 252 °C (dec.)(lit.)
Boiling point 259.73°C (rough estimate)
density 1.694
refractive index 1.5690 (estimate)
storage temp. Store below +30°C.
form Powder
pka 4.41(at 25℃)
color Off-white
Water Solubility 12 g/L cold water
Merck 14,4345
BRN 2050274
Stability: Stability Stable, but may discolour upon exposure to light. Hygroscopic. Incompatible with strong oxidizing agents, strong bases, acid chlorides, acid anhydrides.
InChIKey LNTHITQWFMADLM-UHFFFAOYSA-N
CAS DataBase Reference 149-91-7(CAS DataBase Reference)
NIST Chemistry Reference Benzoic acid, 3,4,5-trihydroxy-(149-91-7)
EPA Substance Registry System Benzoic acid, 3,4,5-trihydroxy-(149-91-7)
Safety Information
Hazard Codes Xi
Risk Statements 36/37/38
Safety Statements 26-36-24/25-37/39
WGK Germany 2
RTECS LW7525000
F 8-9-23
TSCA Yes
HS Code 29182900
Gallic acid Usage And Synthesis
Chemical Properties Colorless crystalline needles or prisms obtained from nutgall tannins,gallic acid is soluble in water and alcohol and melts at 235 to 240 °C. Also known as trihydroxybenzoic acid, it is used in photography, tanning, ink manufacture and pharmaceuticals.
Uses Gallic acid is an important component of iron gall ink, the standard European writing and drawing ink from the 12 th to 19th century with a history extending to the Roman empire and the Dead Sea Scrolls.
Gallic acid Preparation Products And Raw materials
Preparation Products 3,4,5-TRIETHOXYBENZOIC ACID-->1,2,3-Trimethoxybenzene-->Methyl gallate-->Ethyl gallate-->2,3,4-Trimethoxybenzaldehyde-->2,3,4-Trihydroxybenzaldehyde
Raw materials Glycerite-->Gallic acid monohydrate
Tag:Gallic acid(149-91-7)

 China
China

