| Chemical Properties |
red to dark red crystalline powder |
| Uses |
A red glucosidal hydroxyanthrapurin, it is produced naturally within some insects as a defense mechanism. |
| Uses |
antineoplastic, glucosyltransferase inhibitor |
| Definition |
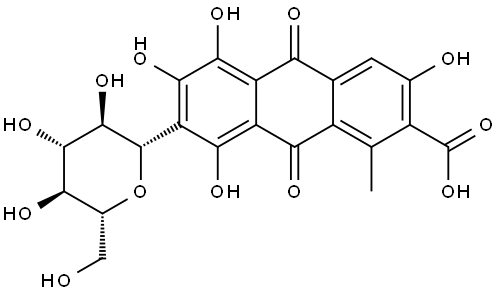
ChEBI: A tetrahydroxyanthraquinone that is that is 1,3,4,6-tetrahydroxy-9,10-anthraquinone substituted by a methyl group at position 8, a carboxy group at position 7 and a 1,5-anhydro-D-glucitol moiety at position 2 via a C-gly osidic linkage. It is a natural dye isolated from several insects such as Dactylopius coccus. |
| General Description |
Dark purplish-brown mass or bright red or dark red powder. Darkens at 248°F. Deep red color in water. Yellow to violet in acidic aqueous solutions. |
| Air & Water Reactions |
Soluble in water [Hawley]. |
| Reactivity Profile |
CARMINE neutralizes bases in exothermic reactions. Incompatible with strong oxidizing agents. |
| Fire Hazard |
Flash point data for CARMINE are not available. CARMINE is probably combustible. |
| Purification Methods |
Carminic acid forms red prisms from EtOH. It gives a red colour in Ac2O and yellow to violet in acidic solution. UV: max (H2O) 500nm ( 6,800); (0.02N HCl) 490-500nm ( 5,800) and (0.0001N NaOH) 540nm ( 3,450). IR: max (Nujol) 1708s, 1693s, 1677m, 1648m, 1632m, 1606s, 1566s, 1509 cm-1. Periodate oxidation is complete after 4hours at 0o with the consumption of 6.2 mols. The tetra-O-methyl carminate has m 186-188o (yellow needles from *C6H6/pet ether). [IR: Ali & Haynes J Chem Soc 1033 1959, Bhatia & Venkataraman Indian J Chem 3 (2) 92 1965, Synthesis: Davis & Smith Biochemical Preparations 4 38 1955, Beilstein 18 III/1V 6697.] |

 China
China