| Brand Name(s) |
Human forms include Omnipen, Principen, Totacillin, Polycillin. Injectable forms include Omnipen-N, Polycillin-N, Totacillin- N.
Veterinary forms include Amp-Equine, and Ampicillin trihydrate (Polyflex). |
| Description |
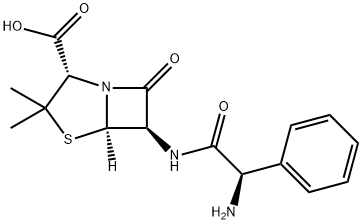
Ampicillin is an antibacterial antibiotic from the α-aminobenzyl penicillin group, which differs from penicillin by the presence of an amino group that facilitates penetration through the outer membrane of some gram-negative bacteria.
Ampicillin is Semi-synthetic derivative of penicillin that functions as an orally active broad-spectrum antibiotic. Ampicillin acts by interfering directly with the biosynthesis of peptidoglycan, which constitutes the major component of the bacterial cell wall, leading to structural instability and death of bacteria.
Ampicillin is a β-lactam antibiotic within the penicillin family. As a member of this family, Ampicillin is susceptible to β-lactamase, which hydrolyzes the β-lactam ring. This broad spectrum antibiotic is effective against gram-positive, gram-negative bacteria and anaerobic bacteria. Ampicillin is widely used in cell culture as a selective agent. As an antibiotic, Ampicillin binds to penicillin binding proteins (PBPs) on a susceptible organism and Inhibits bacterial cell-wall synthesis by inactivating transpeptidases on the inner surface of the bacterial cell membrane. After binding to the PBPs, Ampicilin acts as a structural analogue of acyl-D-alanyl-D-alanine, acylates the transpeptidase enzyme and thereby prevents the cross-linking of the peptidoglycan of the cell wall necessary for the growth of the bacterium.
Ampicillin sodium can be used as a selective agent in several types of isolation media. Ampicillin sodium is routinely used to select for cells containing the pcDNA3.1 and pEAK10 resistance plasmids in cell line A904L at an effective concentration of 50 μg/ml. |
| Chemical Properties |
Crystalline Solid |
| Uses |
β-lactam antibiotics |
| Uses |
Labelled Ampicillin. Orally active, semi-synthetic antibiotic; structurally related to penicillin. Antibacterial. |
| Definition |
ChEBI: A penicillin in which the substituent at position 6 of the penam ring is a 2-amino-2-phenylacetamido group. |
| Brand name |
Amcill (Parke-Davis); Omnipen (Wyeth-Ayerst); Polycillin (Apothecon); Principen (Apothecon). |
| Contact allergens |
Ampicillin caused contact dermatitis in a nurse also sensitized to amoxicillin (with tolerance to oral phenoxymethylpenicillin) and in a pharmaceutical factory worker. Systemic drug reactions are common. Crossreactivity is regular with ampicillin and can occur with other penicillins. |
| Safety Profile |
Poison by intracerebral route. Moderately toxic by intraperitoneal route. Human systemic effects by ingestion: fever, agranulocytosis, and other blood effects. An experimental teratogen. Mutation data reported. Questionable carcinogen. When heated to decomposition it emits very toxic fumes of NO, and SOx,. |

 China
China