| Description |
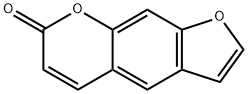
Psoralen is a kind of naturally occurring furocoumarin. It is naturally occurred in the seeds of Psoralea corylifolia as well as in the common fig, celery, parsley, West Indian Satinwood and all citrus fruits. It is capable of binding to DNA via single- and double-strand cross-linking upon photoactivation with UV radiation. It can intercalate with DNA, blocking its synthesis and cell division. For this reason, it is an important mutagen and can be used in related molecular biology studies. It also exhibits anti- proliferative, anti-allergenic and anti-histamine functions. It can also be used in PUVA treatment for skin diseases such as psoriasis, eczema and vitiligo. It is also effective tanning activator in sunscreens. Some of its derivatives, e.g. amino-psoralen, amotosalen HCl, can even inactivate pathogens in blood products. |
| References |
https://en.wikipedia.org/wiki/Psoralen
https://pubchem.ncbi.nlm.nih.gov/compound/Psoralen#section=Top |
| Chemical Properties |
Crystalline Solid |
| Uses |
Use as photochemical probe in biological systems |
| Uses |
Psoralens are phytoalexins; they are used by plants in a defensive response to attacks by fungi and insects. They have also shown photosensitizing and phototoxic effects in animals and humans and have been used in photochemotherapy for management of vitiligo, psoriasis, and mycosis fungoides. Used as photochemical probe in biological systems. |
| Definition |
ChEBI: The simplest member of the class of psoralens that is 7H-furo[3,2-g]chromene having a keto group at position 7. It has been found in plants like Psoralea corylifolia and Ficus salicifolia. |
| Uses |
As photochemical probe in biological systems: P.-S. Song, C.-N. Ou, Ann. N.Y. Acad. Sci. 346, 355 (1980). |

 China
China