| DIRECT BLUE 15 Basic information |
| Product Name: |
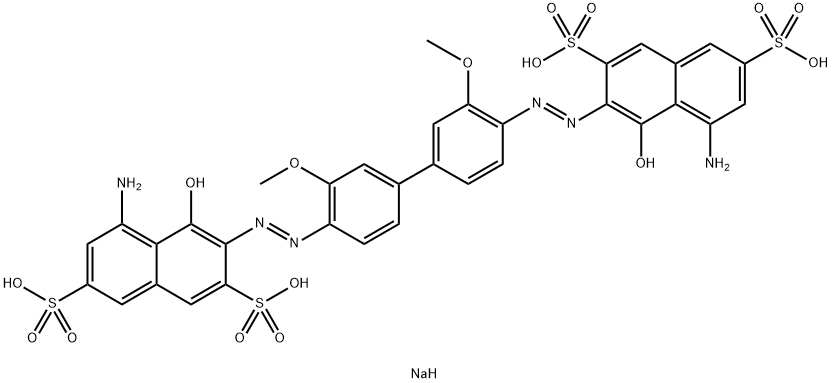
DIRECT BLUE 15 |
| Synonyms: |
kayafectbluey;kayakudirectskyblue5b;mitsuidirectskyblue5b;modrprima15;naphtamineblue10g;ncic61290;nippondirectskyblue;nipponskyblue |
| CAS: |
2429-74-5 |
| MF: |
C34H24N6Na4O16S4 |
| MW: |
992.8 |
| EINECS: |
219-385-3 |
| Product Categories: |
Dyes and Pigments;Organics |
| Mol File: |
2429-74-5.mol |
 |
| |
| DIRECT BLUE 15 Chemical Properties |
| Hazard Codes |
T |
| Risk Statements |
45 |
| Safety Statements |
53-45 |
| RTECS |
QJ6420000 |
| |
| DIRECT BLUE 15 Usage And Synthesis |
| Definition |
ChEBI: An organic sodium salt resulting from the formal condensation of Pontamine sky blue 5B (acid form) with four equivalents of sodium hydroxide. |
| General Description |
Deep purple to dark blue microcrystalline powder. |
| Air & Water Reactions |
Azo dyes can be explosive when suspended in air at specific concentrations. Soluble in water. |
| Reactivity Profile |
DIRECT BLUE 15 is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. |
| Health Hazard |
ACUTE/CHRONIC HAZARDS: When heated to decomposition DIRECT BLUE 15 emits very toxic fumes of nitrogen oxides, sulfur oxides and sodium oxide. |
| Fire Hazard |
Flash point data for DIRECT BLUE 15 are not available; however, DIRECT BLUE 15 is probably combustible. |
| Safety Profile |
A confirmed carcinogen with experimental carcinogenic data reported. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx NazO, and SOx |
|

 China
China







