1/1
Benzoyl peroxide
$ 1.00
/1kg
- Min. Order1 kg
- Purity99%
- Cas No94-36-0
- Supply Ability100KG
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Since:2014-12-17
Address:Zhengzhou High tech Zone, Henan Province, China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Benzoyl peroxide |
| CAS No | 94-36-0 |
| EC-No | |
| Min. Order | 1 kg |
| Purity | 99% |
| Supply Ability | 100KG |
| Release date | 2019/07/06 |
| Benzoyl peroxide Basic information |
| Acne vulgaris Active Ingredients for Acne Medications Mode of action Side effects as Acne TreatmentOther Uses Benzoyl peroxide and Pregnancy References |
| Product Name: | Benzoyl peroxide |
| Synonyms: | Benzaknen;Benzaknew;Benzamycin;Benzoic acid, peroxide;benzoicacid,peroxide;benzoicacidperoxide;Benzol peroxide;Benzoyl |
| CAS: | 94-36-0 |
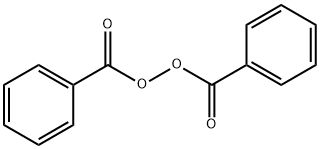
| MF: | C14H10O4 |
| MW: | 242.23 |
| EINECS: | 202-327-6 |
| Product Categories: | Industrial/Fine Chemicals;Organic Peroxide;Synthetic Organic Chemistry;BIOEPIDERM;oxidizing agent |
| Mol File: | 94-36-0.mol |
 |
|
| Benzoyl peroxide Chemical Properties |
| Melting point | 105 °C(lit.) |
| Boiling point | 176°F |
| density | 1.16 g/mL at 25 °C(lit.) |
| refractive index | 1.5430 (estimate) |
| Fp | >230 °F |
| storage temp. | 2-8°C |
| solubility | 0.35mg/l |
| form | powder |
| color | White |
| Water Solubility | Insoluble |
| Merck | 14,1116 |
| BRN | 984320 |
| Stability: | Strong oxidizer. Highly flammable. Do not grind or subject to shock or friction. Incompatible with reducing agents, acids, bases, alcohols, metals, organic materials. Contact with combustible material, heating or friction may cause fire or explosion. |
| InChIKey | OMPJBNCRMGITSC-UHFFFAOYSA-N |
| CAS DataBase Reference | 94-36-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoyl peroxide(94-36-0) |
| EPA Substance Registry System | Peroxide, dibenzoyl(94-36-0) |
| Safety Information |
| Hazard Codes | O,Xn,N,Xi,E,T |
| Risk Statements | 8-36/37/38-43-36-2-7-1-51/53-21/22-62-50-61-3-39/23/24/25-40 |
| Safety Statements | 53-17-26-36/37-45-60-36/37/39-3/7-14A-14-47-35-7-61-37/39-24 |
| RIDADR | UN 3108 5.2 |
| WGK Germany | 2 |
| RTECS | DM8575000 |
| TSCA | Yes |
| HazardClass | 5.2 |
| PackingGroup | II |
| HS Code | 29163200 |
| Hazardous Substances Data | 94-36-0(Hazardous Substances Data) |
| Benzoyl peroxide Usage And Synthesis |
| Acne vulgaris | Although the precise cause of acne is unclear, it appears to be associated with at least four factors: increased sebum production, follicular keratinisation, bacterial colonisation and inflammation. Study suggests the prevalent bacterium implicated in the clinical course of acne is Propionibacterium acnes (P acnes), a gram-positive anaerobe that normally inhabits the skin and is implicated in the inflammatory phase of acne. Benzoyl peroxide is mainly indicated in the treatment of mild to moderate acne and is often prescribed in conjunction with oral antibiotics (erythromycin or clindamycin) in the treatment of moderate to severe acne. |
| Active Ingredients for Acne Medications | Benzoyl peroxide used in 2.5, 5, and 10 percent concentrations, depending on the acne severity. Usually these are in a gel spreading agent, but they can also be in a cream base or a drying paste. Benzoyl peroxide is a keratolytic, which means “keratin-dissolving” and works by loosening dead cells stuck in the follicles. It also releases oxygen in the follicle. Because acne bacteria are anaerobic, they cannot survive in the presence of oxygen. Benzoyl peroxide essentially works both as an interfollicular exfoliant and as an antibacterial. |
| Mode of action | Benzoyl peroxide is lipophilic that can penetrate the stratum corneum and enter the pilosebaceous follicle. It is rapidly broken down to benzoic acid and hydrogen peroxide and generates free radicals that oxidise proteins in bacterial cell membranes, exerting a bactericidal action. In addition, it has shown that benzoyl peroxide can reduce the free fatty acid content of sebum, which provides a useful marker for bacterial activity. Benzoyl peroxide has an anti-inflammatory action and vitro studies suggest that this action arises from its ability to kill polymorphonuclear leukocytes (PMN cells) in the pilosebaceous follicles and so prevent their release of reactive oxygen species such as peroxides which enhance tissue inflammation. Involving equation about this process: C6H5C(O)O-OC(O)C6H5 + H2O 2 C6H5COOH + ½ O2 Moreover, due to its irritant effect, benzoyl peroxide increases turnover rate of epithelial cells, thereby peeling the skin and promoting the resolution of comedones. |
| Side effects as Acne Treatment | Skin reactions such as peeling, itching, irritation, and reddened skin may occur, especially at the start of treatment. A very serious allergic reaction to this drug is rare. This medicine may be harmful if swallowed. |
| Other Uses | 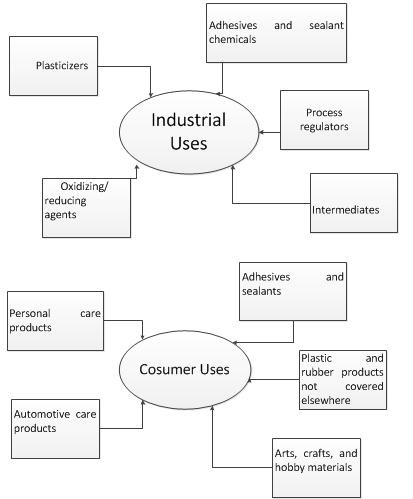 Benzoyl peroxide is used as a bleaching agent for certain foods, an oxidizing agent, a polymerizing initiator in the manufacture of plastics, a curing agent for silicone rubber, and an ingredient in various industrial processes. Benzoyl peroxide, like most peroxides, is a powerful bleaching agent. It has a long history of use in the food industry as a bleaching agent added for flour, whey, and milk for cheese making. Contact with fabrics or hair can cause permanent color dampening almost immediately. Even secondary contact can cause bleaching. Benzoyl peroxide is widely used as a catalyst in the polymerisation of molecules like styrene (phenylethene) to form polystyrene, which used to make many things from drinking cups to packaging material. |
| Benzoyl peroxide and Pregnancy | There are no studies looking at women who use topical benzoyl peroxide during pregnancy. When benzoyl peroxide is applied topically, only 5% is absorbed through the skin, and then it is completely metabolized to benzoic acid within the skin and excreted unchanged in the urine. It is not likely to increase risk for birth defects or cause problems for the baby. However, systemic effects on a pregnant woman and her child would not be expected and therefore use of this product during pregnancy would not be of concern. |
| References | https://medlineplus.gov/druginfo/meds/a601026.html https://pubchem.ncbi.nlm.nih.gov/compound/benzoyl_peroxide#section=Drug-and-Medication-Information http://www.chm.bris.ac.uk/motm/benzoyl-peroxide/benzoylh.htm https://www.webmd.com/drugs/2/drug-1344/benzoyl-peroxide-topical/details https://pubchem.ncbi.nlm.nih.gov/compound/benzoyl_peroxide#section=Top https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3114665/ https://mothertobaby.org/fact-sheets/topical-acne-treatments-pregnancy/pdf/ |
| Chemical Properties | white powder or crystals |
| Uses | vitamin B complex |
| Uses | Benzoyl Peroxide is a widely used organic compound of the peroxide family. Benzoyl Peroxide is often used in acne treatments , bleaching and polymerizing polyester and many other uses. |
| Uses | benzoyl peroxide is an antibacterial ingredient commonly used in acne treatments. It functions by forcing an oxidant (peroxide in this case) into the philosebaceous orifice where it releases oxygen, thereby diminishing the P. acnes population. This reduces the level of free fatty acids and skin infection. Benzoyl peroxide may cause skin irritation in people with sensitive skin. |
| Uses | Source of free radicals for industrial processes. Oxidizing agent in bleaching oils, flour, etc.; catalyst in the plastics industry; initiator in polymerization. |
| Brand name | Acne-Aid Cream (Stiefel); Benoxyl (Stiefel); Benzac (Galderma); Benzac W (Galderma); Brevoxyl (Stiefel); Clear By Design (SmithKline Beecham); Dry and Clear (Whitehall-Robins); Epi-Clear (Bristol-Myers Squibb); Fostex BPO Bar, Gel, and Wash (Bristol-Myers Products); Loroxide (Dermik); PanOxyl (Stiefel); Persa-Gel (Ortho Pharmaceutical); Vanoxide (Dermik). |
| General Description | White, odorless powder, moderately toxic. |
| Air & Water Reactions | Insoluble in water. |
| Contact allergens | Benzoyl peroxide is an oxidizing agent widely employed in acne topical therapy. It is also used as a polymerization catalyst of dental or industrial plastics and as a decolorizing agent of flours, oils, fats, and waxes. Irritant or allergic dermatitis may affect workers in the electronics and plastics (epoxy resins and catalysts) industries, electricians, ceramic workers, dentists and dental technicians, laboratory technicians, bakers, and acne patients. As it was contained in candles, it also induced contact dermatitis in a sacristan. Patch tests may be irritant. |
| Purification Methods | Dissolve benzoyl peroxide in CHCl3 at room temperature and precipitate it by adding an equal volume of MeOH or pet ether. Similarly it is precipitated from acetone by adding two volumes of distilled water. It has also been crystallised from 50% MeOH and from diethyl ether. Dry it under vacuum at room temperature for 24hours. Store it in a desiccator in the dark at 0o. When purifying in the absence of water it can be EXPLOSIVE, and operations should be done on a very small scale with adequate protection. Large amounts should be kept moist with water and stored in a refrigerator. [Kim et al. J Org Chem 52 3691 1987, Beilstein 9 IV 777.] |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;

