| Chemical Properties |
colourless or white crystals |
| Definition |
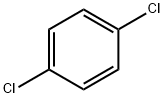
ChEBI: A dichlorobenzene carrying chloro groups at positions 1 and 4. |
| Uses |
Insecticidal fumigant. For domestic use against clothes moths; as space deodorant in room deodorizers, toilet bowl blocks and diaper pail deodorizers. Intermediate in production of plastics for electronic components. |
| General Description |
A white colored liquid with the odor of moth balls. Denser than water and insoluble in water. Flash point below 200°F. Used as a moth repellent, to make other chemicals, as a fumigant, and for many other uses. |
| Air & Water Reactions |
Insoluble in water. |
| Reactivity Profile |
1,4-Dichlorobenzene is incompatible with oxidizing agents. 1,4-Dichlorobenzene is also incompatible with aluminum and its alloys. 1,4-Dichlorobenzene liquefies when mixed with camphor, phenol and salol. 1,4-Dichlorobenzene will attack some forms of plastics, rubber and coatings. . |
| Health Hazard |
INHALATION: irritation of upper respiratory tract; over- exposure may cause depression and injury to liver and kidney. EYE CONTACT: pain and mild irritation. |
| Fire Hazard |
Special Hazards of Combustion Products: Vapors are irritating. Toxic chlorine, hydrogen chloride, and phosgene gases may be generated in fires. |
| Agricultural Uses |
Insecticide, Rodenticide, Fungicide: p-Dichlorbenzene is used primarily as an air deodorant, as moth balls, and in insecticides, which accounts for 90% of the total production of this isomer. Information is not available concerning the production and use of m-DCB. However, it may occur as a contaminant of o-or p-DCB formulations. Both o-and p-isomers are produced almost entirely as byproducts during the production of monochlorobenzene. The major uses of o-DCB are as a process solvent in the manufacturing of toluene diisocyanate and as an intermediate in the synthesis of dyestuffs, herbicides, and degreasers. The para-isomer of dichlorobenzene is the isomer most prominently used in agriculture. Not listed for use in EU countries. |
| Trade name |
DowTHERM®; EVOLA; PARACIDE®; PARA CRYSTALS®; PARADI®; PARADOW®; PARAMOTH®; PARANUGGETS®; PARAZENE®; PERSIA-PERAZOL®; SANTOCHLOR®; Mixed isomers: DILATIN DBI®; MOTTENSCHUTZMITTEL EVAU P®; MOTT-EX®; TOTAMOTT® |
| Safety Profile |
Confirmed carcinogen with experimental carcinogenic data. An experimental teratogen. A human poison by an unspecified route. Moderately toxic to humans by ingestion. Moderately toxic experimentally by ingestion, subcutaneous, and intraperitoneal routes. Other experimental reproductive effects. Human systemic effects by ingestion: unspecified changes in the eyes, lungs, thorax and respiration, and decreased motihty or constipation. Can cause liver injury in humans. A human eye irritant. Mutation data reported. A fumigant. Flammable liquid when exposed to heat, flame, or oxidizers. Dangerous; can react vigorously with oxidizing materials. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, AROMATIC. |
| Purification Methods |
o-Dichlorobenzene is a common impurity. The p-isomer has been purified by steam distillation, crystallisation from EtOH or boiling MeOH, air-dried and dried in the dark under vacuum. It has also been purified by zone refining. [Beilstein 5 IV 658.] |

 China
China