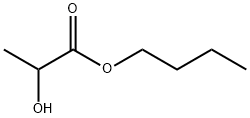
| Description |
Butyl lactate is a kind of lactate derived ester. As a kind of alpha-hydroxy acid ester, its applications in cosmetic, food and pharmaceutical formulations have significantly increased due to their hygroscopic, emulsifying and exfoliating properties. It is used as a food additive because of its flavoring effect. In industry, it can be used as solvent and chemical feedstock. As a bio-based solvent, it can be used as extractant for removing 1-butanol from the aqueous fermentation broths. It can be generally manufactured through the action of lipase from various origins. |
| References |
[1]Zheng, Shaohua, et al. "Feasibility of bio-based lactate esters as extractant for biobutanol recovery:(Liquid+ liquid) equilibria." The Journal of Chemical Thermodynamics 93 (2016): 127-131.
[2]Pirozzi, Domenico, and Guido Greco. "Activity and stability of lipases in the synthesis of butyl lactate." Enzyme and microbial technology 34.2 (2004): 94-100.
[3]Koutinas, Athanasios, et al. "Economic evaluation of technology for a new generation biofuel production using wastes." Bioresource technology 200 (2016): 178-185. |
| Chemical Properties |
CLEAR COLOURLESS LIQUID |
| General Description |
A clear colorless liquid with a mild odor. Flash point 168°F. Less dense than water and insoluble in water. Vapors heavier than air. Used as a solvent, and to make other chemicals. |
| Air & Water Reactions |
Insoluble in water. |
| Reactivity Profile |
Butyl lactate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Avoid contact with strong oxidizing agents and strong bases. Will not polymerize [USCG, 1999]. |
| Health Hazard |
VAPOR: Headache, coughing, possible sleepiness, nausea or vomiting, or dizziness may result. LIQUID: Irritating to skin and eyes. |
| Safety Profile |
Poison by intraperitoneal route. A skin irritant. Toxic concentration in air for humans is about 4 ppm. Flammable when exposed to heat or flame; can react with oxidizing materials. To fight fire, use alcohol foam, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS, n-BUTYL ALCOHOL, and LACTIC ACID. |

 China
China