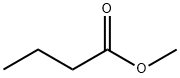
| Description |
Metyhl butyrate occurs naturally in apples, apricot, blackberry, nectarine etc. It is used as a flavor ingredient in fruit and rum flavoring for beverages, ice cream, candy, and baked goods. Methyl butyrate is also used to synthesize butyraldehyde. |
| References |
[1] Ruth Winter, A Consumer's Dictionary of Food Additives, 7th Edition, 2009
[2] Laurent Ducry and Dominique M. Roberge, Dibal-H Reduction of Methyl Butyrate into Butyraldehyde using Microreactors, Organic Process Research & Development, 2008, vol. 12, 163-167 |
| Chemical Properties |
colourless liquid |
| Uses |
manufacture of artificial rum and fruit essences. |
| General Description |
A clear colorless liquid. Flash point 57°F. Less dense than water and slightly soluble in water. Hence floats on water. Vapors heavier than air. |
| Air & Water Reactions |
Highly flammable. Slightly soluble in water. |
| Reactivity Profile |
Methyl butyrate reacts exothermically with acids to generate alcohols and carboxylic acids. Strong oxidizing acids may cause a reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by interaction with basic or caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides . |
| Hazard |
Flammable, dangerous fire risk. |
| Health Hazard |
Irritating to the eyes, nose, throat, upper respiratory tract, and skin. |
| Fire Hazard |
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
| Safety Profile |
Moderately toxic by ingestion and skin contact. A skin irritant. A very dangerous fire hazard when exposed to heat, flame, or oxidizers. Can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS. |
| Purification Methods |
Treat the ester with anhydrous CuSO4, then distil it under dry nitrogen. [Beilstein 2 IV 786.] |

 China
China