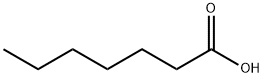
| Description |
As an organic compound, heptanoic acid is a seven-carbon linear chain saturated fatty acid with an unpleasant, rancid and pungent odor, which is commonly used as a chemical intermediate in the synthesis of esters for products, such as ethyl heptanoate, that are used in fragrances and artificial flavors. It is also applied in cosmetics for the production of emollients, skin conditioning agents as well as viscosity controlling agents. Besides, heptanoic acid can also act as a industrial lubricant applied in the fields of aviation, refrigeration, automobile, etc. due to its low viscosity at low temperature and low volatility at high temperature. Moreover, the good anti-corrosion property of heptanoic acid results in the usage of metalworking fluids, industrial water-based refrigerants and anti-corrosion additive for paint. Heptanoic acid is also applied to esterify steroids in the field of pharmaceutical to produce drugs such as testosterone enanthate, trenbolone enanthate, drostanolone enanthate, methenolone enanthate and it is also one of numerous additives in cigarettes. |
| References |
https://en.wikipedia.org/wiki/Heptanoic_acid
https://pubchem.ncbi.nlm.nih.gov/compound/8094#section=Top
http://www.arkema.com/en/products/product-finder/product-viewer/Oleris-n-Heptanoic-acid/ |
| Chemical Properties |
colourless liquid with a pungent and rancid odour |
| Uses |
Intermediates of Liquid Crystals |
| Definition |
ChEBI: A C7, straight-chain fatty acid that contributes to the odour of some rancid oils. Used in the preparation of esters for the fragrance industry, and as an additive in cigarettes. |
| General Description |
A colorless liquid with a pungent odor. Less dense than water and poorly soluble in water. Hence floats on water. Very corrosive. Contact may likely burn skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Flash point near 200°F. |
| Air & Water Reactions |
Slightly soluble in water. |
| Reactivity Profile |
Heptanoic acid reacts exothermically with bases. Can react, particularly if moist, with active metals to form gaseous hydrogen and a metal salt. Such reactions are slow if the acid remains dry. Corrodes or dissolves iron, steel, and aluminum parts and containers under ordinary conditions. Reacts with cyanide salts to generate gaseous hydrogen cyanide, particuarly if moist. May generate flammable and/or toxic gases with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Reacts with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Reacts exothermically with carbonates and bicarbonates to generate a harmless gas (carbon dioxide). Can be oxidized exothermically by strong oxidizing agents and reduced exothermically by strong reducing agents. A wide variety of products is possible. May initiate polymerization reactions; may catalyze chemical reactions. |
| Hazard |
Combustible. |
| Health Hazard |
Harmful if swallowed, inhaled, or absorbed through skin. Extremely destructive to mucous membranes, upper respiratory tract, skin, and eyes. Inhalation may be fatal as a result of spasm, inflammation and edema of the larynx and bronchi, chemical pneumonitis, and pulmonary edema. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting. |
| Fire Hazard |
Heptanoic acid is probably combustible. |

 China
China