| Description |
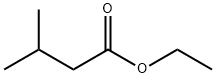
Ethyl isovalerate is the ethyl ester form of isovalerate formed between ethyl alcohol with isovaleric acid. It is a derivative of valeric acid, mainly found in fruits (one of the major component of blueberry). It is a kind of natural food flavoring agent with a fruity type odor and flavor. It is widely used in perfumery and fragrance. It is now frequently synthesized using surfactant-coated lipase (various kinds of origins) immobilized in magnetic nanoparticles. |
| References |
Luebke, William. "ethyl isovalerate108-64-5." (2015).
And, Hiannie Djojoputro, and S. Ismadji. "Density and Viscosity Correlation for Several Common Fragrance and Flavor Esters." Journal of Chemical & Engineering Data 50.2(2005):págs. 727-731.
Mahmood, Iram, et al. "A surfactant-coated lipase immobilized in magnetic nanoparticles for multicycle ethyl isovalerate enzymatic production." Biochemical Engineering Journal 73.8(2013):72-79. |
| Chemical Properties |
clear colorless to pale yellowish liquid |
| Definition |
ChEBI: The fatty acid ethyl ester of isovaleric acid. |
| Uses |
In alcohol solution for flavoring confectionery and beverages. |
| General Description |
A colorless oily liquid with a strong odor similar to apples. Less dense than water. Vapors heavier than air. Flash point 77°F. May mildly irritate skin and eyes. |
| Air & Water Reactions |
Highly flammable. Slightly soluble in water. |
| Reactivity Profile |
ETHYL ISOVALERATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. |
| Health Hazard |
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. |
| Purification Methods |
Wash the ester with aqueous 5% Na2CO3, then saturated aqueous CaCl2. Dry it over CaSO4 and distil. [Beilstein 2 IV 898.] |

 China
China