1/1
Fumaric acid
$ 1.00
/1kg
- Min. Order1 kg
- Purity99.00%
- Cas No110-17-8
- Supply AbilityCARA04
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Since:2014-12-17
Address:Zhengzhou High tech Zone, Henan Province, China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Fumaric acid |
| CAS No | 110-17-8 |
| EC-No | 234-190-3 |
| Min. Order | 1 kg |
| Purity | 99.00% |
| Supply Ability | CARA04 |
| Release date | 2019/07/06 |
| Fumaric acid Basic information |
| Product Name: | Fumaric acid |
| Synonyms: | acidefumarique;Allomaleic acid;allomaleicacid;Boletic acid;boleticacid;Butenedioic acid, (E)-;femanumber:2488;fumaric |
| CAS: | 110-17-8 |
| MF: | C4H4O4 |
| MW: | 116.07 |
| EINECS: | 203-743-0 |
| Product Categories: | INORGANIC & ORGANIC CHEMICALS;FOOD ADDITIVES;Intermediates;Food & Feed ADDITIVES;Substrates;C1 to C5;Carbonyl Compounds;Carboxylic Acids;Alphabetical Listings;E-F;Flavors and Fragrances;Companion Products and ReagentsCancer Research;Chemopreventive Agents;Insect Platform;Multidrug Resistance;Phase II Enzyme Inducers;Phase II Enzyme InducersCancer Research;Serum-free Media;Food & Flavor Additives;Aliphatics;Building Blocks;C1 to C5;Carbonyl Compounds;Carboxylic Acids;Chemical Synthesis;Organic Building Blocks;Allium cepa (Onion);Nutrition Research;Panax ginseng;Phytochemicals by Plant (Food/Spice/Herb);reagent;standard substance;Food additive and acidulant;CONSTRUCTION |
| Mol File: | 110-17-8.mol |
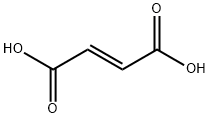 |
|
| Fumaric acid Chemical Properties |
| Melting point | 298-300 °C (subl.)(lit.) |
| Boiling point | 137.07°C (rough estimate) |
| density | 1.62 |
| vapor pressure | 1.7 mm Hg ( 165 °C) |
| refractive index | 1.5260 (estimate) |
| FEMA | 2488 | FUMARIC ACID |
| Fp | 230 °C |
| storage temp. | Store below +30°C. |
| solubility | 95% ethanol: soluble0.46g/10 mL, clear, colorless |
| form | Fine Crystalline Powder |
| pka | 3.02, 4.38(at 25℃) |
| color | White |
| PH | 2.1 (4.9g/l, H2O, 20℃) |
| explosive limit | 40% |
| Water Solubility | 0.63 g/100 mL (25 ºC) |
| Merck | 14,4287 |
| BRN | 605763 |
| Stability: | Stable at room temperature. Decomposes at around 230 C. Incompatible with strong oxidizing agents, bases, reducing agents. Combustible. |
| CAS DataBase Reference | 110-17-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Fumaric acid(110-17-8) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36 |
| Safety Statements | 26 |
| RIDADR | UN 9126 |
| WGK Germany | 1 |
| RTECS | LS9625000 |
| TSCA | Yes |
| HS Code | 29171900 |
| Hazardous Substances Data | 110-17-8(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Fumaric acid Usage And Synthesis |
| Description | Fumaric acid is an important kind of organic chemical raw materials as well as the intermediate of fine chemical products. Meanwhile, it is also an important kind of derivatives of maleic anhydride, being widely used in food, coatings, resins and plasticizers. In the food industry, fumaric acid, used as souring agent, can be applied to soft drinks, western-style wine, cold drinks, fruit juice concentrate, canned fruit, pickles and ice cream. As an acidic substance used as solid beverage gas production agent, it has excellent bubble durability with delicate product organization. Fumaric acid has been used as a food acidulant since 1946. As a food additive, it is used as an acidity regulator and can be denoted by the E number E297. Chemically it is an unsaturated dicarbonic acid and is part of the citric acid cycle. Fumaric acid is a common food additive included in many processed foods to keep them stable and to add tartness. The substance has a more sour flavor than citric acid, another common food additive. Fumaric acid occurs naturally in fumitory, bolete mushrooms, lichen and Iceland moss. As an additive, fumaric acid is produced synthetically, mainly from malic acid from apples. Fumaric acid as an additive is regulated under the Codex Alimentarius General Standard for Food Additives (GSFA), a collection of internationally recognized standards.The U.S. Food and Drug Administration considers it safe. |
| Chemical Properties | Fumaric acid is naturally presented in Corydalis, mushrooms and fresh beef. Product precipitated from the water is monoclinic needle-like, prismatic or leaf-like white crystalline or crystalline powder. It is odorless with a special and strong sour, which is about 1.5 times that of the citric acid. It has a melting point 287 ° C, the boiling point of 290 ° C with subjecting to sublimation at temperature above 200 ° C. When being heated to 230 ° C, it will lose water and become maleic anhydride. Its co-boiling with water can produce DL-malic acid. It is soluble in ethanol, slightly soluble in water and ether, but insoluble in chloroform. The pH value of the 3% aqueous solution is 2.0 to 2.5 with a strong buffering performance, in order to maintain the pH of the aqueous solution at around 3.0. This product is non-toxic; rat-oral LD50: 8000mg/kg. |
| Uses | 1. Fumaric acid is used for the production of unsaturated polyester resin. This kind of resin is characterized by excellent resistance to chemical corrosion as well as heat resistance; the copolymer of fumaric acid and vinyl acetate is a kind of excellent adhesive. Its copolymer with styrene copolymer is the raw material for the manufacture of glass fiber. The plasticizer of the fumaric acid is non-toxic and can be applied to the vinyl acetate latex contact with food. This product is the intermediate of pharmaceutical and optical bleaching agents and other fine chemicals. Neutralization of fumaric acid with sodium carbonate can generate sodium fumarate ([17013-01-3]), and then replaced with ferrous sulfate to get iron fumarate, being the drug Fersamal used for the treatment of small red blood cell anemic. The product, as a food additive-sourness agent, used in soft drinks, fruit sugar, jelly, ice cream with most of them used in combination with sourness agent, citric acid. The monosidum salt made from the reaction between fumaric acid and sodium hydroxide can also used as sour seasoning, also used as the intermediate of synthetic resin and mordant. 2. Fumaric acid is included in many dairy-based products. These include dairy drinks such as chocolate milk, cocoa, eggnog, condensed milk and whey protein beverages. It also may be added to clotted cream, milk and cream powders and milk and cream analogues (substitutes). Fumaric acid is added to cheese products, including processed cheese and cheese substitutes. Dairy-based desserts, such as pudding, flavored yogurt, sherbet and sorbet may include fumaric acid as well. Dairy fat spreads and blended spreads can include fumaric acid, and so can preserved eggs and egg-based desserts such as custard. 3. Some processed and packaged foods have fumaric acid added to them to help stabilize them and enhance their flavor. For example, many processed meats, such as bacon and canned meats, have added fumaric acid. Frozen seafood, smoked meats and the edible casings around sausages might also have fumaric acid added to them. Fermented, canned, dried and processed fruits and vegetables can contain the food additive as well. Rice cakes and other precooked rice foods, dried or preserved eggs, mustard, vinegar, cider, wine and other alcoholic beverages are additional examples of foods that might contain fumaric acid. |
| Uses | Occurs in many plants. Essential to vegetable and tissue respiration. Used as an antioxidant. |
| Uses | fumaric acid is used to add fragrance to products and to decrease product pH. It can also help keep the pH stable. It is generally used in cleansers. Fumaric acid is naturally occurring in plants, such as lichen and Iceland moss, and in animals. For example, the skin produces fumaric acid when exposed to light. It can also F be synthetically manufactured. |
| Definition | ChEBI: A butenedioic acid in which the C2C double bond has E geometry. It is an intermediate metabolite in the citric acid cycle. |
| General Description | A colorless crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Combustible, though may be difficult to ignite. Used to make paints and plastics, in food processing and preservation, and for other uses. |
| Air & Water Reactions | Slightly soluble in water. |
| Reactivity Profile | Fumaric acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Fumaric acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. Partial carbonization and formation of maleic anhydride occur at 446° F (open vessel). |
| Health Hazard | Inhalation of dust may cause respiratory irritation. Compound is non-toxic when ingested. Prolonged contact with eyes or skin may cause irritation. |
| Purification Methods | Crystallise it from hot M HCl or water and dry it at 100o. [Beilstein 2 IV 2202.] |
| Fumaric acid Preparation Products And Raw materials |
| Preparation Products | L-Alanine-->L-Aspartic acid -->Succinic acid-->D-Tartaric acid-->Dimethyl fumarate-->1-Boc-4-(4-methoxycarbonylphenyl)piperazine-->4-[4-(tert-Butoxycarbonyl)piperazino]benzoic acid-->SEMOTIADIL-->Fumaryl chloride-->6-Methylcoumarin-->DL-Malic acid-->FUMARONITRILE-->Ferrous fumarate-->BROMOSUCCINIC ACID-->Disodium fumarate-->NEBRACETAM-->Diethyl fumarate -->Benzylfumarate |
| Raw materials | Benzene-->Thiocarbamide-->Maleic anhydride -->Activated carbon-->Sodium chlorate-->Mineral oil-->D(+)-Sucrose-->Maleic acid-->Ammonium bromide-->Maltose-->butene -->Isomerization catalyst-->wax liquid |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;

