| Indications and Usage |
Vildagliptin (vildagliptin), developed by Novartis (Novartis) Pharmaceutical Co., Ltd, is another oral administrated inhibitor of Dipeptidyl peptidase-IV after sitagliptin (sitagliptin). In 2008, it is approved for marketing in the European Union for the treatment of type 2 diabetes.
Diabetes is a chronic metabolic disease with its prevalence being increased year by year. Type 2 diabetes is a complicated diseases caused with the combined action between polygenic genetic factors and environmental factors.
Dipeptidyl peptidase IV (DPP-IV) inhibitors are a new class of anti-diabetic drugs which induces and facilitate the biosynthesis and secretion of insulin. It plays a role in lowering the blood carbohydrate concentration through multiple mechanisms such as inhibiting the β cell apoptosis, inhibiting glucagon secretion and reducing food intake. During its lowering effect on reducing blood carbohydrate levels, it can also reverse the situation of deteriorating function of pancreas islet for diabetic patients at the same time. Vildagliptin is the representative of drugs among the dipeptidyl peptidase inhibitor. It exhibited a good anti-diabetic effects and tolerance no matter whether it is being administered alone or in combination with metformin and insulin medication in clinical studies. |
| Mechanisms of Action |
Vildagliptin is a selective, competitive and reversible DPP-4 inhibitor. Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 21 (GLP21) are important hormones that regulate body glucose concentration and have the same functions as intestinal insulin. Type-2 diabetes patients suffer from GIP insulin secretion failure, so only their GLP21 can promote insulin secretion by affecting receptors on islet beta cell membranes. GLP21 can also inhibit glucagon secretion and inhibit gastric clearing to extend the feeling of fullness (control appetite). DPP-4 binds with proteins in many types of tissues, including tissue in the kidney, liver, small intestine brush border, pancreatic duct, lymph cells, and endothelial cells, and it can deactivate GLP21 by hydrolyzing its N-terminal position-2 alanine. Vildagliptin binds with DPP-4 to produce a DPP-4 compound and inhibit its activity. It increases GLP21 concentration and promotes islet beta cells to produce insulin, while also lowering glucagon concentration, thus lowering blood sugar. Vildagliptin has not noticeable effects on weight. |
| Adverse reactions |
Most common adverse reactions include headache, nasopharyngitis, coughing, constipation, dizziness and excess sweating. There have been very rare cases of hypotension, but it is unclear if conditions are related to this drug. Almost all research shows that the hypoglycemia occurrence rate while using Vildagliptin is similar to the rate when using a placebo. Control trials showed that compared to thiazolidinediones, Vildagliptin causes a similar occurrence rate of headaches, rashes, and other adverse reactions, but Vildagliptin has a lower occurrence rate of adverse reactions forcing patients to cease treatment and of severe adverse reactions. |
| Chemical Properties |
White Solid |
| Uses |
Vildagliptin (LAF-237) inhibits DPP?4 with IC50 of 2.3 nM |
| Uses |
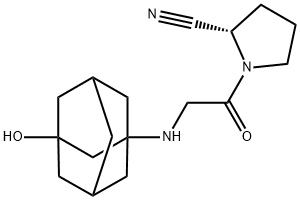
The major metabolite of Vildagliptin |
| Uses |
Labelled Vildagliptin. It is a new oral anti-hyperglycemic agent of a new dipeptidyl peptidase-IV (DPP-IV) inhibitor class of drugs. Antidiabetic. |
| Uses |
A metabolite of Vildagliptin |

 China
China