| Chemical properties |
It appears as white crystalline or crystalline powder, being almost odorless and hygroscopic. Its color will change in case of light. It is easily soluble in water, slightly soluble in ethanol, but insoluble in ether and benzene. |
| Application |
Biochemical research; clinical drugs belong to fat-soluble vitamins; it is clinically used as a hemostatic drug.
Vitamin K3 is mainly used as poultry feed enhancer at a dosage of 1-5mg/kg.
The goods can have addition reaction with sodium bisulfite to generate vitamin K3.
VK3. Used as raw material of feed additives; it can mainly promote the liver synthesis of prothrombin in livestock and poultry, and promote the liver synthesis of plasma coagulation factors as a hemostatic agent.
Traits: bright yellow crystal with very spicy smell. It is stable in the air and will be decomposed in sunlight. 1G can be dissolved in about 60ml ethanol, 10ml benzene and 50 ml vegetable oil. It is soluble in chloroform and carbon tetrachloride but insoluble in water. The ethanol solution was neutral to litmus paper. The solution will not be decomposed even when heated to 120 °C. It will be destroyed upon treatment with alkali and reducing agent. It is toxic with the half lethal dose (mouse, oral) being about 500 mG/kG. It is irritating. Its commodities still include sodium bisulfite manaquinone, appearing as white crystalline powder. It has no smell or with slightly special smell. It has hygroscopicity. It undergoes decomposition in case of light to turn into yellow or purple color. It is easily soluble in water, slightly soluble in ethanol, but almost insoluble in ether and benzene. Application: biochemical research |
| Production |
There are two production processes. 1. Methyl naphthalene is obtained from the oxidization of chromic anhydride. 2-methyl naphthalene is dissolved in glacial acetic acid, stirred and cooled to temperature below 40 ℃. Slowly add the mixture of chromic anhydride and the same amount of water so that the temperature can be maintained at 35-40 ℃. After the completion of the addition, maintain the temperature at 40 ℃ for 0.5 h, the temperature was then raised to 70 ℃ for 45min, and further heated to 85 ℃ for 15min. Pour the reactants into a lot of water and stir continuously to precipitate out the 2-naphthoquinone. Filter and rinse the filter cake repeatedly with water, until the aqueous solution has no sour. Filter to get the 2-menenoquinone with a yield of 51%. 2-methyl naphthalene can also be made from sodium dichromate and potassium dichromate with the oxidation yield being roughly the same. 2. Cyclohexanone has cyclization reaction with butadiene to get 2-methyl naphthalene hydroquinone, followed by oxidation with chromic acid to obtain the final product. Dissolve the toluquinone in glacial acetic acid; send the butadiene to the required amount at temperature below 20 ℃; stand for 20 hours; heat to release the remaining butadiene and continue to heat to about 110 °C for refluxing of 3 hours. Then recycle 30% of glacial acetic acid through vacuum distillation and cooled to temperature below 40 ℃; slowly add the mixture of chromic acid and the same amount of water so that the temperature is maintained at 65-70 ℃. After the completion of the addition, maintain the temperature at 70-80 degree for 1h to obtain the menadione.
O-methyl naphthoquinone is used as raw material. It undergoes oxidation with glacial acetic acid and chromic anhydride, followed by addition with sodium bisulfite in ethanol to derive it. |
| Chemical Properties |
Bright yellow crystals |
| Uses |
Precursor to verious types of Vitamin K. Used as a micronutrient for livestock and pet foods. |
| Uses |
prothrombogenic agent |
| Uses |
antibacterial |
| Uses |
The primary known function of vitamin K is to assist in the normal clotting of blood, but it may also play a role in normal bone calcification. |
| Definition |
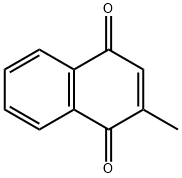
ChEBI: A member of the class of 1,4-naphthoquinones that is 1,4-naphthoquinone which is substituted at position 2 by a methyl group. |

 China
China