1/1
D-Biotin
$ 1.00
/1KG
- Min. Order1G
- Purity98%
- Cas No58-85-5
- Supply Ability100KG
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Since:2014-12-17
Address:Zhengzhou High tech Zone, Henan Province, China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | D-Biotin |
| CAS No | 58-85-5 |
| EC-No | |
| Min. Order | 1G |
| Purity | 98% |
| Supply Ability | 100KG |
| Release date | 2019/07/06 |
AD68
| D-Biotin Basic information |
| Description Physicochemical property Physiological function Biotin and fat metabolism Biotin deficiency Food source Toxicity Side effect Distinguishing test Content analysis Applications References |
| Product Name: | D-Biotin |
| Synonyms: | RONACARE(TM) BIOTIN PLUS;PHOTOPROBE(R) BIOTIN;VITAMIN B7;VITAMIN H;BIOTIN(V-H);BIOTINUM;BIOTIN, IMMOBILIZED ON DEXTRAN-LYSINE;BIOTIN, IMMOBILIZED ON DEXTRAN |
| CAS: | 58-85-5 |
| MF: | C10H16N2O3S |
| MW: | 244.31 |
| EINECS: | 200-399-3 |
| Product Categories: | cyclic compounds;PHARMACEUTICALS;Miscellaneous Natural Products;Other Reagents;Biochemistry;Vitamins;Nutritional Supplements;Vitamins and derivatives;Biotin Derivatives;Intermediates & Fine Chemicals;Vitamin Ingredients;API;Vitamin series;Inhibitor;Biotinylation Reagents;RONIACOL;Isolabel;biotinyl;Inhibitors |
| Mol File: | 58-85-5.mol |
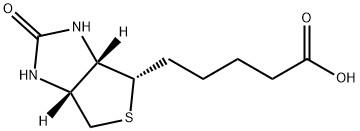 |
|
| D-Biotin Chemical Properties |
| Melting point | 231-233 °C(lit.) |
| alpha | 89 º (c=1, 0.1N NaOH) |
| density | 1.2693 (rough estimate) |
| refractive index | 90.5 ° (C=2, 0.1mol/L NaOH) |
| storage temp. | 2-8°C |
| solubility | H2O: 0.2 mg/mL Solubility increases with addition of 1 N NaOH. |
| form | powder |
| PH | 4.5 (0.1g/l, H2O) |
| Water Solubility | Soluble in hot water, dimethyl sulfoxide, alcohol and benzene. |
| Sensitive | Light Sensitive |
| Merck | 14,1231 |
| BRN | 86838 |
| Stability: | Stable, but light sensitive. Incompatible with strong oxidizing agents, strong bases, strong acids, formaldehyde, chloramine-T, nitrous acid. |
| InChIKey | YBJHBAHKTGYVGT-ZKWXMUAHSA-N |
| CAS DataBase Reference | 58-85-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Biotin(58-85-5) |
| EPA Substance Registry System | 1H-Thieno[3,4-d] imidazole-4-pentanoic acid, hexahydro-2-oxo-, (3aS,4S,6aR)-(58-85-5) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-36/37/38 |
| Safety Statements | 24/25-36-26 |
| WGK Germany | 1 |
| RTECS | XJ9088200 |
| F | 8 |
| TSCA | Yes |
| HS Code | 29362930 |
| Hazardous Substances Data | 58-85-5(Hazardous Substances Data) |
| D-Biotin Usage And Synthesis |
| Description | D-Biotin, also called Vitamin H, is a colorless, water-soluble member of the group of B-vitamins. Formerly it was known as vitamin H or coenzyme R. It has many benefits for the hair, skin, and nails. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring. Biotin is a coenzyme for carboxylase enzymes, involved in the synthesis of fatty acids, isoleucine, and valine, and in gluconeogenesis. Subclinical deficiency of Biotin can cause mild symptoms, such as hair thinning or skin rash typically on the face. Thus, This product is recommended for general food fortification and dietary supplement applications. Generally, D-Biotin can be used for baby food and dietetics, for solid and liquid pharmaceutical preparations, for cosmetic preparations, and for use in the fermentation industry. |
| Physicochemical property | Biotin is widely distributed in animals and plants, and the natural presence of biotin is mainly in the form of binding with other molecules. The biochemical structure of biotin includes a shuttle chain containing five carbon atoms and two five-membered heterocycles. In vivo the shuttle of the side chain binds with lysine s residue of enzyme protein, playing a role of coenzyme. Biotin may have 8 different isomers, of which only D-biotin has biological activity. Under normal circumstances, biotin is quite stable, only in the strong acid, alkali, formaldehyde and UV treatment will be destroyed. Biotin is the carrier of carboxyl in the carboxylation reaction required large ATP. The carboxyl group is temporarily bound to a nitrogen atom on the bicyclic ring system of biotin, such as in the reaction of pyruvate carboxylase catalyzing the pyruvate carboxylation of oxaloacetate.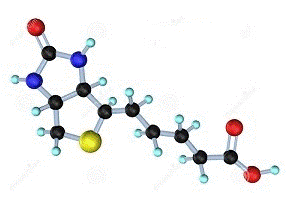 Figure1: The structural formula of the biotin molecule |
| Physiological function | Biotin is necessary for cell growth, the production of fatty acids, and the metabolism of fats and amino acids. It plays a role in the citric acid cycle, which is the process by which biochemical energy is generated during aerobic respiration. Biotin is a coenzyme for carboxylase enzymes, involved in the synthesis of fatty acids, isoleucine, and valine, and in gluconeogenesis. In addition, biotin is widely used throughout the biotechnology industry to conjugate proteins for biochemical assays. We need biotin about 100 to 300 micrograms per day. There is an antibiotic protein that could combine with biotin in the egg white egg. After combining, it cannot be absorbed by the digestive tract; resulting in animal biotin deficiency, at the same time loss of appetite, glossitis, dermatitis dermatitis, hair removal and so on. However, there is no case of biotin deficiency on human, probably because in addition to food sources, intestinal bacteria can also synthesize biotin. Biotin is a coenzyme of a lot of enzymes in the human body. It participates in the metabolism of aliphatic acid, carbohydrate, vitamin B12, folic acid and pantothenic acid; promoting synthesis of protein and urea, and also promoting excretion.
|
| Biotin and fat metabolism | As a coenzyme of phthalocyanine coenzyme A, Biotin is involved in the synthesis of fatty acids, catalyzing the formation of glycine CoA. The reaction is the first step in the synthesis of fatty acids, and then through the cytoplasmic multi-enzyme complex and fatty acid synthase synthesize palmitic acid from the phthalocyanine-CoA. In the prolongation of carbon chain in the fatty acid, the propanedio phthalide-ACP is involved in the reaction as a donor of the dicarbon unit, and the dipropyl phthalide-ACP is derived from the phthalide-CoA. It can be seen that biotin is necessary for the synthesis of fatty acids and the extension of carbon chains in fatty acid. Biotin deficiency can lead to abnormal lipid metabolism, resulting in changes of fatty acid composition in the body. In addition, synthesis of saturated fatty acid decreased, synthesis of triglyeeride increased, and fat in the liver and kidney increased by 2 to 5 times. Biotin-deficient diets increase the rate of desiccation of palmitic acid in liver tissue by five folds. Palmitic acid increased, at the same time stearic acid reduced. Biotin is also a necessary material for synthesis of long-chain unsaturated fatty acid and fatty acid metabolism. Biotin is also associated with the synthesis of acetylcholine and the metabolism of cholesterol. The lack of biotin reduces the ability of animals to synthesize arachidonic acid from linoleic acid, leading to the accumulation of linoleic acid in the body. |
| Biotin deficiency | Biotin deficiency appears to be rare, but some groups may be more susceptible.Biotin supplements are widely available but rarely necessary. A deficiency can lead to:
Other symptoms may include:
|
| Food source |
Processing food reduces levels of nutrients such as biotin, so raw cauliflower, for example, would provide more biotin than cooked cauliflower. A study published in Advances in Nutrition estimates biotic intake in North America and Western Europe at between 35 to 70 μg per day, or 143 to 287 mmol per day. According to Oregon State University, biotin is not known to cause toxic effects. People with hereditary disorders of biotin metabolism tolerate doses of up to 200,000 mcg per day without any problems. Individuals with no biotin metabolism disorder who took doses of 5,000 mcg per day for 24 months had no adverse effects. However, it is important to speak to a physician or dietitian before making any change to nutritional intake or using supplements. |
| Toxicity | Toxicity of biotin seems to be low. Treatment of seborrheic dermatitis with high doses of biotin did not detect abnormal protein metabolism or genetic errors and other metabolic abnormalities. Animal experiments also show that biotin toxicity is low. |
| Side effect | Bursts of cystic acne of jaw and chin are the most common side effects of biotin. The specific reason is not very clear. And usually this symptom will disappear by itself after a few weeks. There are also some nutritional supplements who report that acne symptoms can be reduced when the dose is limited to 2500 micrograms or less. In short, the situation experienced by each person is slightly different. Life-threatening biotin is extremely rare. Eosinophilic pleural effusion is the only documented deadly case that causes a woman to die. She ingested a lot of biotin, along with vitamin B5. So far, it is unclear whether her death is caused by biotin, B5, or a mixture of both. Healthy adults are less likely to develop biotin deficiency. This disease is common in excessive consumption of avidin (which can be found in raw eggs), or people with skin or hair disorders (such as phenylketonuria). The symptoms of lacking biotin usually take several years to show. The study found that about 50% of pregnant women had a biotin deficiency problem. They lack a kind of enzyme to tell the body how to use biotin properly, which leads to a decline in metabolic function. Most physicians do not recommend biotin nutrition as a treatment because the trial for rats found that it had a risk of miscarriage and fetal defects. Biotin is often recommended as nourishment for the promotion of hair and nail health. Because of the impact on carbohydrates, it is also often used to control body weight. Biotin supplements are known for their beauty and metabolic effects. The recommended dose ranges from 3 micrograms to 5000 micrograms per day, depending on the specific use. The side effects are relatively rare, and it is relatively easy to control even if the side effects appear, so it is a safe nutritional supplements. |
| Distinguishing test | The warm water saturated solution of the sample could cause the drop of the bromine test solution (TS-46) to fade. |
| Content analysis | Accurately weighed the sample about 500mg, mixed with 100ml of water, plus phenolphthalein test solution (TS-167) a few drops, with 0.1mol/L sodium hydroxide solution slowly added to the suspension in continuous heating and stirring, until we got pink suspension. 0.1 mol/L sodium hydroxide per ml is equivalent to 24.43 mg biotin (C10Hl6N2O3S). |
| Applications |
|
| References | https://www.ulprospector.com/en/na/PersonalCare/Detail/473/317131/D-Biotin https://en.wikipedia.org/wiki/Biotin http://www.medicalnewstoday.com/articles/219718.php http://www.selleckchem.com/products/biotin-vitamin-b7.html |
| Chemical Properties | White powder |
| Uses | vasodilator |
| Uses | vitamin B complex |
| Uses | Growth factor present in minute amounts in every living cell. Plays an indispensable role in numerous naturally occurring carboxylation reactions. Occurs mainly bound to proteins or polypeptides. The richest sources are liver, kidney, pancreas, yeast, and milk. The biotin content of cancerous tumors is higher than that of normal tissue. |
| Definition | ChEBI: An organic heterobicyclic compound that consists of 2-oxohexahydro-1H-thieno[3,4-d]imidazole having a valeric acid substituent attached to the tetrahydrothiophene ring. The parent of the class of biotins. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;


