| Vitamin K |
In 1929, a famous chemist Dam (Denmark) had first discovered and extracted a yellow crystalline substance from animal liver and linseed oil-vitamin K.
Vitamin K is a the indispensable substance for hepatic synthesis of the four kinds of coagulation factors (active clotting factor Ⅱ (prothrombin), coagulation factor Ⅶ, coagulation factors Ⅺ and coagulation factor Ⅹ). Upon a lack of vitamin K1, the above four coagulation factors synthesized in liver synthesis become abnormal protein molecules while their ability to catalyze clotting effect dropped significantly. It is known that vitamin K is the cofactor for the glutamic acid γ-carboxylation. Lack of vitamin K disables the above γ-carboxylation of coagulation factors; in addition, these types of blood coagulation factors will reduce and will cause blood clotting slowing down and bleeding disorders. In addition, it is generally recognized that vitamin K was dissolved in mitochondrial membrane lipids, playing a role of electron transfer. Vitamin K can increase the intestinal motility and secretion property. Lack of vitamin will cause reduction in the tension and contraction of smooth muscle and can also affect the metabolism of some hormones, such as causing delay of the decomposition of glucocorticoids in the liver. It also has similar effects as hydrocortisone. Long-term injection of vitamin K can increase the thyroid and other endocrine activity.
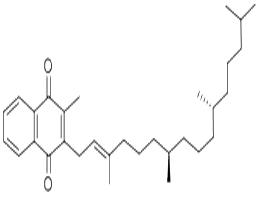
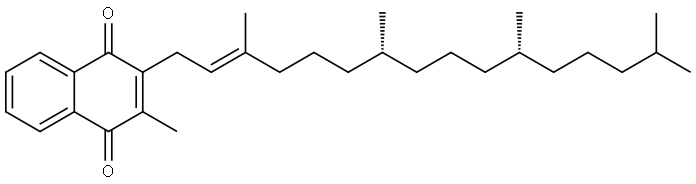
Vitamin K can be divided into two categories with both of them belonging to 2-methyl-1, 4-naphthoquinone derivatives. One class belongs to fat-soluble vitamins, namely vitamin K1 extracted from green plants (such as alfalfa, spinach and other leafy greens, etc.) and vitamin K2 extracted from microorganisms. Vitamin K2 can also be synthesized by human intestinal bacteria (such as E. coli). Vitamin K1 appears as yellow oil-like liquid while K2 appears pale yellow crystals. Both of them have heat resistance property, but are vulnerable to ultraviolet radiation to be destroyed, and therefore should be stored separately. Another class is water-soluble vitamins, namely artificially synthetic vitamin K3 and K4. The most important vitamins are K1 and K2.
Vitamin K appears as oily liquid or solid and is insoluble in water but soluble in oils and organic solvents such as ether. It is chemically stable with heat and acid resistant, but being susceptible to alkali and UV degradation. The human body has a low demand. However, newborn infants are vulnerable to being lack of vitamin K that is an important vitamin for promoting blood clotting and normal bone growth. Dark green vegetables and yogurt are easily available vitamin K supplements obtained from the daily diet. Human has a very small demand for vitamin K but it is needed to maintain the normal function of blood coagulation, reduce heavy bleeding menstrual period, but also to prevent internal bleeding and hemorrhoids. Patients suffering frequent nosebleeds should be take more vitamin K from natural foods.
This information is edited by Xiongfeng Dai from Chemicalbook. |
| Limited use |
Limited GB 14880-94: baby food, 420~475 μg/ku. |
| Chemical Properties |
It appears as yellow to orange transparent viscous liquid and is odorless with the relative density being 0.967 and the refractive index being nD251.525~1.528. It is easily soluble in chloroform, ether and vegetable oil, slightly soluble in ethanol and insoluble in water. It is easily to be decomposed when exposed to light and with being decomposed upon being heated to 120 ℃. |
| Uses |
1. It can be used as food supplements. It can be used in infant foods with the usage amount being 420~475μg/kg.
2. It belongs to vitamin drugs to be used for the prevention and treatment of vitamin K1 deficiency symptom, low thrombin disease and natural newborn hemorrhagic disease.
3. promote blood clotting.
4. promote the synthesis of primary liver thrombin.
5. increase the intestinal motility and secretion function. |
| Production method |
It can be obtained through the following process: o-naphthoquinone is mixed with acetic anhydride to have reduction, acetylation reaction to generate acetylated menadione in the presence of zinc; followed by hydrolysis in ammonia and further condensation reaction with phytol in ether with the catalysis of boron trifluoride to generate dihydro vitamin K1; and finally with hydrolysis, oxidation, purification and refining to derive the products. |
| Chemical Properties |
Yellow Oil |
| Uses |
vitamin K1 |
| Uses |
Labeled Phytonadione, intended for use as an internal standard for the quantification of Phytonadione by GC- or LC-mass spectrometry. |
| Uses |
Occurs widely in green plants, algae, photosynthetic bacteria. |

 China
China