1/1
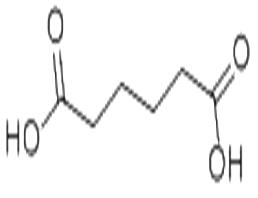
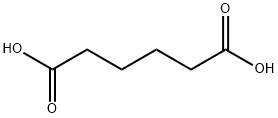
Adipic acid
$2.30 10000KG
$3.40 100KG
- Min. Order100KG
- Purity99%
- Cas No124-04-9
- Supply Ability200T
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Since:2014-12-17
Address:Zhengzhou High tech Zone, Henan Province, China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Adipic acid |
| CAS No | 124-04-9 |
| EC-No | 204-673-3 |
| Min. Order | 100KG |
| Purity | 99% |
| Supply Ability | 200T |
| Release date | 2019/07/06 |
CR337
| Product Name: | Adipic acid |
| Synonyms: | RARECHEM AL BO 0180;AKOS BBS-00004308;ADIPIC ACID;adipinic acid;1,6-HEXANEDIOIC ACID;1,4-BUTANEDICARBOXYLIC ACID;BUTANE-1,4-DICARBOXYLIC ACID;DICARBOXYLIC ACID C6 |
| CAS: | 124-04-9 |
| MF: | C6H10O4 |
| MW: | 146.14 |
| EINECS: | 204-673-3 |
| Product Categories: | Industrial/Fine Chemicals;alpha,omega-Alkanedicarboxylic Acids;alpha,omega-Bifunctional Alkanes;Monofunctional & alpha,omega-Bifunctional Alkanes;Food additive and acidulant;plasticizer |
| Mol File: | 124-04-9.mol |
 |
|
| Adipic acid Chemical Properties |
| Melting point | 151-154 °C(lit.) |
| Boiling point | 265 °C100 mm Hg(lit.) |
| density | 1,36 g/cm3 |
| vapor density | 5 (vs air) |
| vapor pressure | 1 mm Hg ( 159.5 °C) |
| FEMA | 2011 | ADIPIC ACID |
| Fp | 385 °F |
| storage temp. | Store below +30°C. |
| solubility | methanol: 0.1 g/mL, clear, colorless |
| pka | 4.43(at 25℃) |
| form | Solid |
| color | White |
| PH | 2.7 (23g/l, H2O, 25℃) |
| Water Solubility | 1.44 g/100 mL (15 ºC) |
| Merck | 14,162 |
| BRN | 1209788 |
| Stability: | Stable. Substances to be avoided include ammonia, strong oxidizing agents. |
| InChIKey | WNLRTRBMVRJNCN-UHFFFAOYSA-N |
| CAS DataBase Reference | 124-04-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Hexanedioic acid(124-04-9) |
| EPA Substance Registry System | Hexanedioic acid(124-04-9) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36-41 |
| Safety Statements | 26-39-24/25 |
| RIDADR | UN 9077 |
| WGK Germany | 1 |
| RTECS | AU8400000 |
| TSCA | Yes |
| HS Code | 29171210 |
| Hazardous Substances Data | 124-04-9(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| 1,4-Butanedicarboxylic acid | English |
| SigmaAldrich | English |
| ACROS | English |
| Adipic acid Usage And Synthesis |
| As a flavor ingredient | Identification
Its aqueous solutions have the lowest acidity of any of the common food acids. For concentrations from 0.5 to 2.4 g/100 mL, the pH of its solution varies less than half a unit. Hence, it can be used as a buffering agent to maintain acidities within the range of 2.5 to 3.0. This is highly desirable in certain foods, yet the pH is low enough to inhibit the browning of most fruits and other foodstuffs. Regulatory Status: CoE: Approved FDA: 21 CFR 131.111, 184.1009, 582.1009 FDA (other): n/a JECFA:ADI: 0–5 mg/kg bw (1977). No safety concern when used at current levels as a flavoring agent (1999). Reported uses (ppm): (FEMA, 1994)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Properties | white crystalline powder | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses | progestin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses | Adipic Acid is primarily used in the synthesis of nylon. It has been used as a reagent in the solid-state polymerization of nylon analogs. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Definition | ChEBI: An alpha,omega-dicarboxylic acid that is the 1,4-dicarboxy derivative of butane. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Description | Adipic acid is a white crystalline solid. Adipic acid is insoluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Adipic acid is used to make plastics and foams and for other uses. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Air & Water Reactions | Dust may form explosive mixture with air [USCG, 1999]. Insoluble in water. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactivity Profile | Adipic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Adipic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. Behavior in Fire: Melts and may decompose to give volatile acidic vapors of valeric acid and other substances. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Hazard | Inhalation of vapor irritates mucous membranes of the nose and lungs, causing coughing and sneezing. Contact with liquid irritates eyes and has a pronounced drying effect on the skin; may produce dermatitis. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fire Hazard | Behavior in Fire: Melts and may decompose to give volatile acidic vapors of valeric acid and other substances. Dust may form explosive mixture with air. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adipic acid Preparation Products And Raw materials |
| Preparation Products | Cyclopentanone-->Polyurethane foams-->1,5-Pentanedioic acid-->Triethylene glycol dimethacrylate-->1,6-HEXANEDIAMINE-->1,6-Diisocyanatohexane-->Glutaric anhydride-->LIGNOCERIC ACID-->Fluorescent Brightener 184-->Monostearin-->Cyclopentanol-->1,4-Dicyanobutane-->PolyesterPolyol-->2,5-Thiophenedicarboxylic acid-->Diethyl adipate-->2,2-DIMETHYLCYCLOPENTANONE-->Adipoyl chloride-->NYLON 6-->polyesters containing quarternary ammonium groups-->NYLON 6/6-->Dioctyl adipate-->Water-soluble resin-->Diethyl 2,5-dibromohexanedioate-->Dibutyl adipate -->Bis(2-ethylhexyl) adipate-->Dimethyl adipate-->Monoethyl Adipate |
| Raw materials | Sodium hydroxide-->Nitric acid-->Copper-->Cyclohexane-->Cyclohexanone-->1,3-BUTADIENE-->Cupric acetate monohydrate -->Cyclohexanol-->Vanadium pentoxide -->Cyclohexene-->Bromide-->MANGANESE(II) ACETATE-->METABORIC ACID |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;