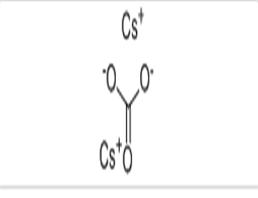
Cesium carbonate
$ 100.00
/25KG
- Min. Order25KG
- Purity99%
- Cas No534-17-8
- Supply Ability100tons
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Cesium carbonate |
| CAS No | 534-17-8 |
| EC-No | 208-591-9 |
| Min. Order | 25KG |
| Purity | 99% |
| Supply Ability | 100tons |
| Release date | 2019/07/06 |