Outline
Luliconazole is a novel topical antifungal imidazole, and is a kind of analogue of lanoconazole. It can interfere with the fungal cell wall synthesis and fungal growth by decreasing levels of ergosterol via inhibiting lanosterol demethylase activity. Besides being used for the treatment of athlete's foot, jock itch and ringworm, it has also been developed for onychomycosis (nail fungus) treatment and has now also entered the clinical stage phase III. This product was originally developed by the Japanese pesticide Corporation (NihonNohyaku Co., Ltd.). In November 2013, the FDA has approved a 1% luliconazole cream for entering into market for topical treatment of interdigital athlete's foot, jock itch and ringworm with the trade name being Luzu and first entered into market in North America. As early as April 2005, luliconazole had been approved to enter into market in Japan under the trade name Lulicon. In January 2010 and June 2012, it was approved for marketing in India and China, respectively.
In 1997, Pesticide Co., Ltd. of Japan has initially get access to the worldwide patent of luliconazole as antifungal agents (WO 1997002821 A2) and have protected in their preparation and application; thereafter, it had also applied for a European patent (EP0839035 A2), Chinese patent (CN 1194582 A) and U.S. Patent (US5900488A). In addition, WO 2007102241, US 8058303, and other patents have also been applied for protection on the drug's pharmaceutical compositions and dosage forms.
Synthetic Method
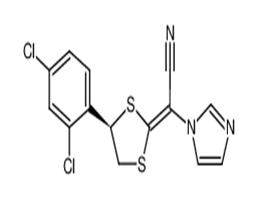
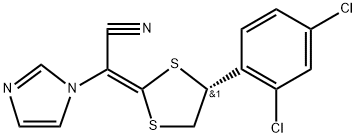
The first method is using BH3/THF and a kind of chiral catalyst for stereoselectively reducing the starting material 1 to give the intermediate 2 which yields the corresponding mesylate intermediate (3), substance 3 and intermediate 5 is cyclized into luliconazole in the presence of potassium hydroxide and DMSO; second method is based on using chiral compound 6 as starting materials, 6 undergoes mesylate esterification to yield active intermediate 7,7 undergoes condensation reaction with intermediate 5 to get luliconazole. The synthesis of Intermediate 5 is through putting 2-(1-imidazolyl)-acetonitrile and CS2 into condensation under basic conditions.
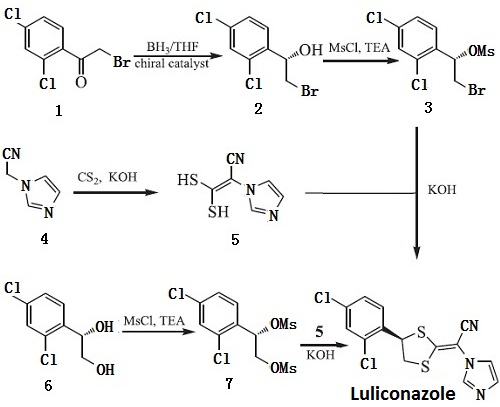
Figure 1 is a synthesis route of luliconazole
Application
It can be used for the following fungal infections:
Ringworm: athlete's foot, ringworm, jock itch;
Candida infections: disease fingers erosion, intertrigo; vitiligo.
Currently, ringworm treatment drugs include two major categories: first, propylene amine drugs, such as terbinafine, Butenafine and naftifine. They exert their bactericidal effects through inhibiting squalene cyclase, causing the lack of ergosterol and accumulation of squalene. The second category of imidazole (imidazoles) drugs: such as miconazole, econazole, clotrimazole, ketoconazole and bifonazole. They are a class of synthetic antifungal agent that can selectively inhibit the lanosterol 14α-demethylation activity of fungal cell, preventing the ergosterol synthesis of cell membrane, changing the cell membrane permeability, and resulting in the loss of important intracellular fungal material and causing fungal death. Imidazole antifungal agents are currently the most commonly used drugs in clinical treatment of ringworm with extensive clinical applications.
In vitro and in vivo studies have shown that luliconazole has broad-spectrum antifungal activity, with its minimum inhibitory concentration (MIC) being 0.12 to 2 mg/mL to Trichophyton (Trichophyton rubrum, Trichophyton mentagrophytes and tonsurans). Its anti-fungal effect is stronger than terbinafine, ketoconazole, miconazole, bifonazole and other commonly used drugs. Trichophyton rubrum is most sensitive to luliconazole. The MIC of Luliconazole to Candida albicans is 0.031~0.130μg/mL with the inhibitory effect being higher than that of terbinafine, Liranaftate, Butenafine, amorolfine and bifonazole, but less than that of ketoconazole, clotrimazole, neticonazole and miconazole. The MIC of Luliconazole on the important pathogens of seborrheic dermatitis, limiting Malassezia is very low, being 0.004~0.016 μg/mL with its inhibitory effect being not less but even stronger than ketoconazole.
In addition, luliconazole also have antifungal activity on filamentous fungi and yeast-like fungi with its strength being comparable as lanoconazole but higher than bifonazole and terbinafine, but being almost ineffective on Zygomycetes.

 China
China