Top Quality 99% Ciclesonide 126544-47-6 for Allergic Rhinitis and Asthma Treatment
Ciclesonide Basic Info
Ciclesonide Product name: Ciclesonide
Ciclesonide Synonyms: (r)-11b,16a,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with cyclohexanecarboxaldehyde 21-isobutyrate;(R)-11b,16a,17,21- Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with cyclohexanecarboxaldehyde 21-isobutyrate;Ciclesonide;(11β,16α)-16,17-[[(R)-CyclohexylMethylene]bis(oxy)]-11-hydroxy-21-(2-Methyl-1-oxopropoxy)-pregna-1,4-diene-3,20-dione;Ciclesonide(RPR251526)
Ciclesonide CAS No.: 126544-47-6
Ciclesonide Assays: 99%
Ciclesonide M.F.: C32H44O7
Ciclesonide M.W.: 540.69
Ciclesonide Melting point:202-209°C
Ciclesonide Appearance: White Powder
Ciclesonide Packing: 100G/foil bag
Ciclesonide Reference Standard: Pharmaceutical grade
Ciclesonide Applicant: Mainly for allergic rhinitis and asthma treatment, can effectively control and eliminate respiratory allergies.
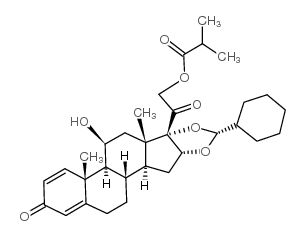
Description: Ciclesonide is a glucocorticoid used to treat asthma and allergic rhinitis. It is marketed under the brand name Alvesco for asthma and Omnaris/Omniair for hay fever in the US and Canada. Phase 3 trials for the hay fever indication outside the US are ongoing. The drug was approved for adults and children 12 and over by the US Food and Drug Administration in October 2006. Side effects of the medication include headache, nosebleeds, and inflammation of the nose and throat linings.


 China
China


