| CAS: | 583-61-9 |
| MF: | C7H9N |
| MW: | 107.15 |
| EINECS: | 209-514-1 |
| Product Categories: | Biochemistry;Reagents for Oligosaccharide Synthesis;Pyridines derivates;Heterocyclic Compounds;bc0001 |
| Mol File: | 583-61-9.mol |
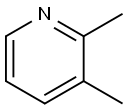 |
|
| 2,3-Lutidine Chemical Properties |
| Melting point | -15 °C (lit.) |
| Boiling point | 162-163 °C (lit.) |
| density | 0.945 g/mL at 25 °C (lit.) |
| refractive index | n20/D 1.508(lit.) |
| Fp | 122 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| pka | 6.57(at 25℃) |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Water Solubility | 95 g/L (26 ºC) |
| BRN | 106418 |
| InChIKey | HPYNZHMRTTWQTB-UHFFFAOYSA-N |
| LogP | 1.638 (est) |
| CAS DataBase Reference | 583-61-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyridine, 2,3-dimethyl-(583-61-9) |
| EPA Substance Registry System | 2,3-Dimethylpyridine (583-61-9) |
| 2,3-Lutidine Usage And Synthesis |
| Chemical Properties | clear colorless to slightly yellow liquid |
| Uses | 2,3-Lutidine is a lansoprazole intermediates. It is used as pharmaceutical intermediate. |
| Synthesis Reference(s) | The Journal of Organic Chemistry, 66, p. 53, 2001 DOI: 10.1021/jo000724t |
| Purification Methods | Steam distil it from a solution containing about 1.2 equivalents of 20% H2SO4, until ca 10% of the base has been carried over with the non-basic impurities. The acidic solution is then made alkaline, and the base is separated, dried over NaOH or BaO, and fractionally distilled. The distilled lutidine is converted to its urea complex by stirring 100g with 40g of urea in 75mL of H2O, cooling to 5o, filtering at the pump, and washing with 75mL of H2O. The complex, dissolved in 300mL of H2O, is steam distilled until the distillate gives no turbidity with a little solid NaOH. The distillate is then treated with excess solid NaOH, and the upper layer is removed: the aqueous layer is then extracted with diethyl ether. The upper layer and the ether extract are combined, dried (K2CO3), and distilled through a short column. Final purification is by fractional crystallisation using partial freezing. The picrate crystallises from EtOH with m 187-188o. [Kyte et al. J Chem Soc 4454 1960, Beilstein 20 H 243, 20 II 159, 20 III/IV 2765, 20/6 V 15.] |
Product picture
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
