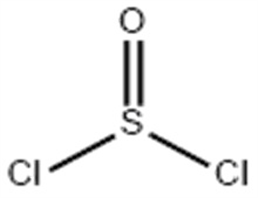
Molecular formula: Cl2OS
Appearance traits: transparent to yellow liquid
Storage conditions:
Storage precautions Store in a cool, ventilated warehouse. The storage temperature does not exceed 30℃, and the relative humidity does not exceed 75%. Keep the container tightly closed. It should be stored separately from alkalis, etc., and avoid mixed storage. The storage area should be equipped with leakage emergency treatment equipment and suitable storage materials.
Stability:
1. It is easily decomposed into sulfur dioxide and hydrogen chloride in contact with water. Soluble in benzene, chloroform and carbon tetrachloride. When heated to 140°C, it begins to decompose to produce chlorine, sulfur dioxide and sulfur monochloride. The chlorine atom has a remarkable ability to replace hydroxyl or mercapto groups, and sometimes it can replace sulfur dioxide, hydrogen or oxygen. Thionyl chloride can react with hydroxyl-containing phenol or alcoholic organic compounds to form the corresponding chloride, react with sulfonic acid to form sulfonyl chloride, and react with Grignard reagent to form the corresponding sulfoxide compound. It has strong refractive power and has an unpleasant smell like SO2. Thionyl chloride will decompose significantly at a temperature slightly higher than the boiling point, and the decomposition products are S2Cl2, SO2 and Cl2. It is soluble in benzene and chloroform, and reacts with water to generate SO2 and HCl.
2. Very lively and highly corrosive. Its vapor and liquid have a strong stimulating effect on eyes, mucous membranes and skin. Wear protective gloves during operation to avoid skin contact. Thionyl chloride must be stored in a glass bottle in a dry environment at room temperature. It reacts with water and emits toxic gases HCl and SO2. Since most of the reactions that SOCl2 participates in will emit the above two gases, it must be operated in a fume hood. SOCl2 will decompose to produce Cl2, SO2 and S2Cl2 when it is higher than 140 oC. Impurities of iron and/or zinc will catalyze and aggravate the decomposition reaction.
3. Stability and stability
4. Incompatible materials water, alkalis
5. Conditions to avoid contact with hot, humid air
6. Polymerization hazards, no polymerization
Welcome to contact us and look forward to cooperating with you!!!
Contact details:
Whatsapp / Skype / WeChat: +8613373414058
Email: gantuo02@cngantuo.com

 China
China