1/2
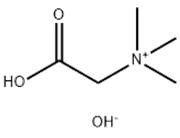
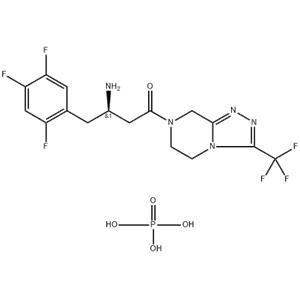
Sitagliptin phosphate monohydrate NEW
- Min. Order1KG
- Purity99%
- Cas No654671-77-9
- Supply Abilityg-kg-tons, free sample is available
- Update time2024-03-29

| Product Name | Sitagliptin phosphate monohydrate |
| CAS No | 654671-77-9 |
| EC-No | |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | g-kg-tons, free sample is available |
| Release date | 2024/03/29 |
1. Product information
Names
| Name | Sitagliptin phosphate monohydrate |
|---|---|
| Synonym | More Synonyms |
Sitagliptin phosphate monohydrate Biological Activity
| Description | Sitagliptin phosphate monohydrate is a potent inhibitor of DPP4 with IC50 of 19 nM in Caco-2 cell extracts |
|---|---|
| Related Catalog | Signaling Pathways >> Autophagy >> Autophagy Signaling Pathways >> Metabolic Enzyme/Protease >> Dipeptidyl Peptidase Research Areas >> Metabolic Disease |
| Target | IC50: 19 nM (DPP4, in Caco-2 cell extracts) |
| In Vitro | Sitagliptin phosphate exhibits a potent inhibitory effect on DPP-4 with IC50 of 19 nM from Caco-2 cell extracts[1]. Sitagliptin reduces in vitro migration of isolated splenic CD4 T-cells through a pathway involving cAMP/PKA/Rac1 activation[2]. A recent study demonstrates that sitagliptin exerts a novel, direct action in order to stimulate GLP-1 secretion by the intestinal L cell through a DPP-4-independent, protein kinase A- and MEK-ERK1/2-dependent pathway. It therefore reduces the effect of autoimmunity on graft survival[3]. |
| In Vivo | In vivo, the ED50 value of sitagliptin phosphate for inhibition of plasma DPP-4 activity is calculated to be 2.3 mg/kg 7 hour postdose and 30 mg/kg 24 hour postdose in freely fed Han-Wistar rats[1]. The streptozotocin-induced type 1 diabetes mouse model exhibits elevated DPP-4 levels in the plasma that can be substantially inhibited in mice on an Sitagliptin phosphate diet. This is achieved by a positive effect on the regulation of hyperglycemia, potentially through prolongation of islet graft survival[4]. The plasma clearance and volume of distribution of Sitagliptin phosphate are higher in rats (40-48 mL/min/kg, 7-9 L/kg) than in dogs (9 mL/min/kg, 3 L/kg); and its half-life is shorter in rats,2 hours compared with 4 hours in dogs[5]. |
| Kinase Assay | DPP-4 is extracted from confluent Caco-2 cells. After 5 minutes of incubation at room temperature with lysis buffer (10 mM Tris-HCl, 150 mM NaCl, 0.04 U/mL aprotinin, 0.5% Nonidet P40, pH 8.0), cells are centrifuged at 35,000 g at 4°C for 30 minutes, and the supernatant is stored at -80°C. Assays are performed by mixing 20 μL of appropriate compound dilutions with 50 μL of the substrate for the DPP-4 enzyme, H-Ala-Pro-7-amido-4-trifluoromethylcoumarin (final concentration in the assay, 100 μM) and 30 μL of the Caco-2 cell extract (diluted 1000-fold with 100 mM Tris-HCl, 100 mM NaCl, pH 7.8). Plates are incubated at room temperature for 1 hour, and fluorescence is measured at excitation/emission wavelengths of 405/535 nm using a SpectraMax GeminiXS. Dissociation kinetics of inhibitors from the DPP-4 enzyme is determined after a 1-hour preincubation of Caco-2 cell extracts with high inhibitor concentrations (30 nM for BI 1356, 3 μM for vildagliptin). The enzymatic reaction is started by adding the substrate H-Ala-Pro-7-amido-4-trifluoromethylcoumarin after a 3000-fold dilution of the preincubation mixture with assay buffer. Under these conditions, the difference in DPP-4 activity at a certain time point in the presence or absence of an inhibitor reflects the amount of this inhibitor still bound to the DPP-4 enzyme. Maximal reaction rates (fluorescence units/seconds ×1000) at 10-minute intervals are calculated using the SoftMax software of the SpectraMax and corrected for the rate of an uninhibited reaction [(vcontrol-vinhibitor)/vcontrol]. |
| Cell Assay | CD4T-cells are plated on membrane inserts in serum-free RPMI 1640, and cell migration is assayed using Transwell chambers (Corning), in the presence or absence of purified porcine kidney DPP-4 (32.1 units/mg; 100 mU/mL final concentration) and DPP-4 inhibitor (100 μM). After 1 hour, cells on the upper surface are removed mechanically, and cells that have migrated into the lower compartment are counted. The extent of migration is expressed relative to the control sample. |
| Animal Admin | Mice: Overnight fasted C57BL/6J mice are challenged 45 min after compound administration with an oral glucose load (2 g/kg). Blood samples for glucose measurement are obtained by tail bleed predose and at serial time points after the glucose load. To evaluate the duration of the effect on glucose tolerance, vehicle or DPP-4 inhibitors are administered 16 h before the glucose challenge. |
| References | [1]. Thomas, L., et al. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylm ethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of acti [2]. Kim, S.J., et al., Dipeptidyl peptidase IV inhibition with MK0431 improves islet graft survival in diabetic NOD mice partially via T-cell modulation. Diabetes, 2009. 58(3): p. 641-51. [3]. Sangle, G.V., et al., Novel biological action of the dipeptidylpeptidase-IV inhibitor, sitagliptin, as a glucagon-like peptide-1 secretagogue. Endocrinology, 2012. 153(2): p. 564-73. [4]. Kim, S.J., et al., Inhibition of dipeptidyl peptidase IV with sitagliptin (MK0431) prolongs islet graft survival in streptozotocin-induced diabetic mice. Diabetes, 2008. 57(5): p. 1331-9. [5]. Beconi, M.G., et al. Disposition of the dipeptidyl peptidase 4 inhibitor sitagliptin in rats and dogs. Drug Metab Dispos, 2007. 35(4): p. 525-32. |
Chemical & Physical Properties
| Boiling Point | 529.9ºC at 760 mmHg |
|---|---|
| Molecular Formula | C16H20F6N5O6P |
| Molecular Weight | 523.324 |
| Flash Point | 274.3ºC |
| Exact Mass | 523.105530 |
| PSA | 173.84000 |
| LogP | 1.66180 |
Safety Information
| Hazard Codes | Xn |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
Synthetic Route
Previous 1/3 Next Sitagliptin CAS#:486460-32-6 ~95% 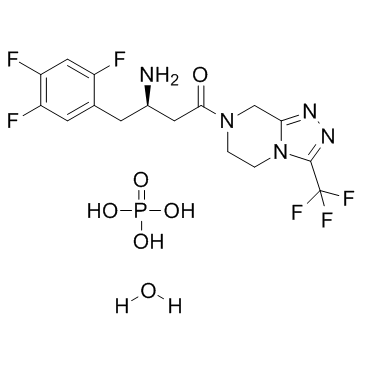 Sitagliptin pho... CAS#:654671-77-9 |
| Literature: Lin, Kuaile; Cai, Zhengyan; Zhou, Weicheng Synthetic Communications, 2013 , vol. 43, # 24 p. 3281 - 3286 |
![(2Z)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one structure](https://www.chemsrc.com/caspic/261/764667-65-4.png) (2Z)-4-Oxo-4-[3... CAS#:764667-65-4 ~%  Sitagliptin pho... CAS#:654671-77-9 |
| Literature: Synthetic Communications, , vol. 43, # 24 p. 3281 - 3286 |
![(2Z)-1-[5,6-Dihydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazin-7(8H)-yl]-3-[[(1R)-1-phenylethyl]amino]-4-(2,4,5-trifluorophenyl)-2-buten-1-one structure](https://www.chemsrc.com/caspic/462/1169707-29-2.png) (2Z)-1-[5,6-Dih... CAS#:1169707-29-2 ~%  Sitagliptin pho... CAS#:654671-77-9 |
| Literature: Synthetic Communications, , vol. 43, # 24 p. 3281 - 3286 |
Precursor & DownStream
| Precursor 7 | Previous 1/2 Next |
|---|---|
| |
| DownStream 0 | |
Customs
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
Synonyms
| 7-[(3R)-3-Amino-1-oxo-4-(2,4,5-trifluorophenyl)Butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazinephosphatemonohydrate |
| 3(R)-3-amino-1-[3-(trifluoromethyl)-5H,6H,7H,8H [1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one phosphate monohydrate |
| Sitagliptin phosphate for research |
| 1-Butanone, 3-amino-1-[5,6-dihydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazin-7(8H)-yl]-4-(2,4,5-trifluorophenyl)-, (3R)-, phosphate, hydrate (1:1:1) |
| Januvia |
| (3R)-3-Amino-1-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-4-(2,4,5-trifluorophenyl)-1-butanone phosphate hydrate (1:1:1) |
| Sitagliptin phosphate hydrate (jan) |
| Sitagliptin phosphate hydrate |
| (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine dihydrogenphosphate monohydrate |
| Sitagliptin phosphate (usan) |
| Sitagliptin Phosphate |
| Januvia (tn) |
| Sitagliptin monohydrate |
| (3R)-3-Amino-1-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-4-(2,4,5-trifluorophenyl)butan-1-one phosphate hydrate (1:1:1) |
| Sitagliptin (phosphate monohydrate) |
| Acetylcysteine Impurity 3 |
2. Packaging
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping

4. Contact information
For more details, pls contact us freely.
Email address : Sun@fdachem.com
Mob: 86 13526505137
WhatsApp/Skype/Wechat/LINE: 86 13526505137
Company Profile Introduction
Henan Fengda Chemical Co., Ltd. is located in the High-tech Development Zone of Henan Province. Specializing in the production and sales of various fine chemical products required for industrial production, including chemical raw materials, organic raw materials, petrochemicals, chemical reagents, solvents, catalysts, and additives, etc.

 CAS#:868071-17-4
CAS#:868071-17-4