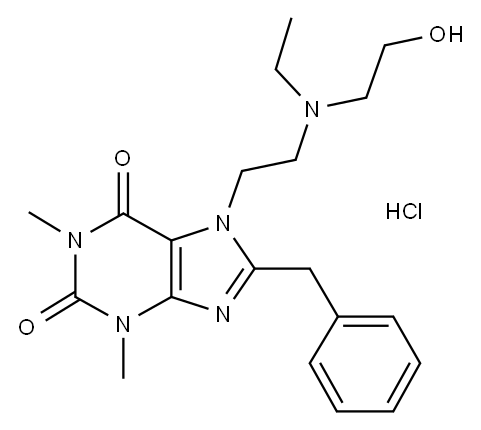
A mixture of 100 kg of 8-benzyltheophilline, 36 L of N-ethylethanolamine, 300
L of 1,2-dichlorethane and 71 kg of sodium carbonate was refluxed for 24
hours. Then 36 L of N-ethylethanolamine was added and the reaction mixture
was refluxed. After cooling to the mixture was added the water and
hydrochloric acid. The organic phase was extracted with hydrochloric acid. The
acidic phase was neutralized with sodium carbonate and the 7-(N-ethyl-N-β-
hydroxyethylaminoethyl)-8-benzyltheophilline was extracted with
dichloromethane. The solvent was evaporated and the free base of 7-(N-ethyl-
N-β-hydroxyethylaminoethyl)-8-benzyltheophilline was dissolved in methanol.
Hydrochloride of 7-(N-ethyl-N-β-hydroxyethylaminoethyl)-8-benzyltheophilline
was obtained by addition to the solution the hydrochloric acid; yield 81%,
melting point 185-186°C.