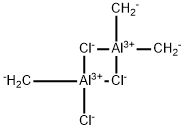
The aluminum alkyl halides are flammable, reactive, and may be spontaneously combustible in air. They are colorless to yellow liquids. Ethylaluminum dichloride:(563-43-9):
Catalyst for polymerization of olefins, catalyst
for hydrogenation of aromatics.
Ignites spontaneously in air, reacts violently
with water, keep out of contact with air and moisture.
A flammable liquid.
Danger from spontaneous combustion.
When heated to decomposition it emits
toxic fumes of Cl-.
These materials are used as components of olefin polymerization catalysts. The reader is referred to the entry on “Aluminum alkyls” for additional information on this entry. The aluminum alkyl halides parallel very closely the aluminum alkyls
UN3052 Spontaneously combustible. Water reactive releasing large quantities of toxic and deadly hydrogen gas. (Note: this number does not appear in the 49/CFR HazMat tables)
The aluminum alkyl halides are strong reducing agents; they react—possibly violently—with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. These chemicals react violently with nitromethaneEthylaluminum sesquichloride reacts explosively with carbon tetrachloride at room temperature. This chemical reacts violently with water, forming corrosive hydrogen chloride and flammable ethane gas. Diethylaluminum chloride may form an explosive product with chlorine azide.