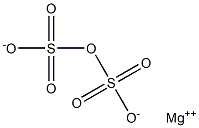
Magnesium pyrosulfate has the molecular formula of
MgS2O7 and the molecular weight of 200.4328 g/mol.
Almost no data concerning this salt is available either
in patents, scientific or technical literature, or in reference
books. Since this salt is not stable in water, it
must be prepared by a solid-state reaction. One such
reaction involves heating ammonium pyrosulfate with
the oxide:
MgO+(NH4)2S2O7+heat ? MgS2O7+2H2O+2NH3
It is known that the lower molecular weight alkaline
earth pyrosulfates are not very stable in moist air or
aqueous solution and hydrolyze to sulfate:
S2O72-+H2O ? 2SO42-+2H+
MgS2O7+H2O?2MgSO4+2H+
Magnesium pyrosulfate, once formed, is relatively
stable in dry air and can be heated to about 750 °C before
it decomposes to the sulfate.