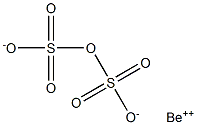
Beryllium pyrosulfate is not stable in aqueous solution
and so must be prepared via a solid-state reaction.
It would have the molecular formula of BeS2O7 and the molecular weight of 185.1401 g/mol. It could be
prepared by the reaction involving “fuming” sulfuric
acid (oleum) with beryllium sulfate:
[Be(OH)]2SO4+(H2SO4)n ? 2BeS2O7+(n+2) H2O
The [Be(OH)]2SO4 salt is added to the oleum and
allowed to dissolve in an excess of acid. The acid is
heated to 150–175°C to drive off the water. This is
an equivalent of using H2S2O7. The solution is allowed
to equilibrate and crystals of the anhydrous salt
appear. These are separated and allowed to dry. Beryllium
disulfate is stable and may be heated to a red
heat before it begins to decompose to the sulfate
and SO3.