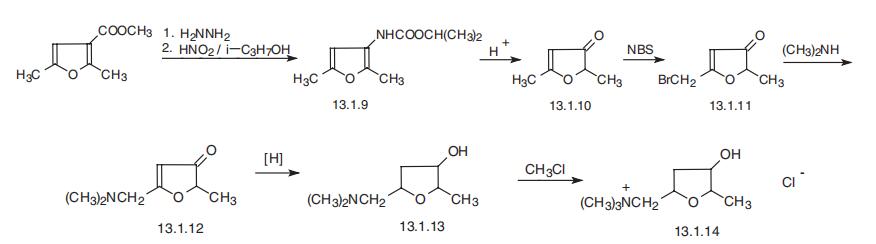
(+/-)-MUSCARINE CHLORIDE
- Product Name(+/-)-MUSCARINE CHLORIDE
- CAS2936-25-6
- MFC9H20ClNO2
- MW209.71
- EINECS
- MOL File2936-25-6.mol
Chemical Properties
| storage temp. | room temp |
| solubility | deionized water: ≥20mg/mL |
| form | powder |
| color | white |
| Water Solubility | deionized water: ≥20mg/mL |
Safety Information
| Hazard Codes | Xn |
| Risk Statements | 22-36 |
| Safety Statements | 26-36 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| HazardClass | 6.1(a) |
| PackingGroup | II |