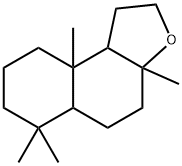
3a,6,6,9a-Tetramethyldodecahydronaphtho[2,1-b]furan is a crystalline autoxidation product of
ambrein with a typical ambergris odor. It is prepared
from (?)-sclareol, a diterpene alcohol obtained from extraction of clary sage
plants. Oxidative degradation to a lactone (“sclareolide”),
hydrogenation of the latter to the corresponding diol, and dehydration yield the
title compound.
Reported found in clary sage oil.
This ingredient provides warmth, richness and elegance to all areas of perfumery from sheer florals to modern ambers. Due to its power, best effects can be achieved at lower dosages, however, higher levels are often used to provide performance and substantivity.
Racemic sclareolide can be prepared by cyclization of homofarnesic acid in the
presence of SnCl4 as a catalyst . Pure diastereomers are obtained by acid
cyclization of (E)- and (Z)-4-methyl-6-(2,6,6-trimethylcyclohex-l(2)-enyl)-3-
hexen-1-ol, prepared from 2-methyl-4-(2,6,6-trimethylcyclohex-l(2)-enyl)-2-
butenal [394]. If the racemic sclareolide mixture is resolved into its enantiomers,
the (–)-oxide may also be obtained by a totally synthetic route
ChEBI: Ambronide is a naphthofuran.
Compound starting from natural sclareol: Ambermore,
Ambermore-DL, Ambermore-EX (Aromor), Ambrox® Super (Firmenich),
Ambroxan® (Kao), Ambroxide (Symrise); compound starting from homofarnesic
acid derivatives: Ambrox® DL (Firmenich); compound starting from 2-methyl-
4-(2,6,6-trimethylcyclohex-l(2)enyl)-2-butenal: Cetalox® (Firmenich), Cetalor
(Aromor).