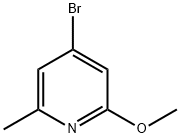
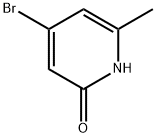
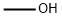General procedure for the synthesis of 4-bromo-2-methoxy-6-methylpyridine from methanol and 4-bromo-6-methylpyridin-2-ol: Methanol (54 μL, 1.3 mmol), triphenylphosphine (419 mg, 1.60 mmol), and diisopropyl diazocarboxylate (323 mg, 1.60 mmol) were sequentially added to a solution of 4-bromo-6-methylpyridin-2-ol (200 mg, 1.60 mmol) in THF (4.6 mL) under the protection of nitrogen gas. 1.60 mmol), triphenylphosphine (419 mg, 1.60 mmol) and diisopropyl diazocarboxylate (323 mg, 1.60 mmol). The reaction mixture was stirred at room temperature for 18 hours. After completion of the reaction, it was diluted by adding ethyl acetate and washed (3 times) with 1N NaOH solution. The organic layer was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by rapid chromatography on silica gel with the eluent ethyl acetate/hexane (5% to 10% gradient) to afford 4-bromo-2-methoxy-6-methylpyridine (65 mg, 0.32 mmol, 30% yield).