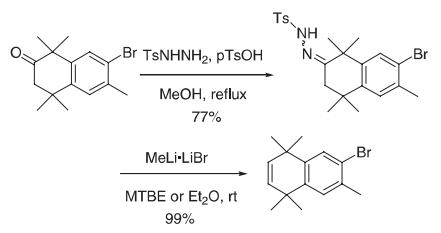
A suspension of the ketone (30.5 g, 0.10 mol), p-toluenesulfonylhydrazide (22.1 g, 0.119 mol), and p-tolunesulfonic acid monohydrate (4.92 g, 25. 9 mmol) in MeOH (611 mL) was heated at reflflux under N2 for 24 h. The resultant reaction slurry was cooled for 1 h in an ice-bath, fifiltered, and rinsed with cold MeOH (150 mL) to give 36.8 g (77%) of the hydrazone.
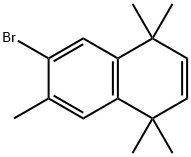
(Caution: this transformation produces nitrogen gas. The reaction system should be vented. Do not run in a closed system! This reaction may also be run with diethyl ether.) A suspension of the hydrazone (20.0 g, 43.2 mmol) in methyl tert-butylether (MTBE 400 mL) was treated with a solution of MeLi as a complex with LiBr (1.5 M in Et2O, 86.3 mL, 0.13 mol) at room temperature for 1 h, cooled to 0 °C, and quenched with water (500 mL). The reaction was extracted with MTBE (1 L), the organic layer was dried (MgSO4), and the solvent was removed in vacuo to give 12.0 g (99%) of the alkene as a white solid.