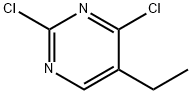
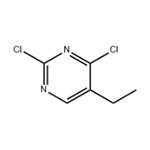
2,4-Dichloro-5-ethylpyrimidine is a reactant used for the synthesis of pyrimidine derivatives as inhibitors of dipeptidyl peptidase IV, TBK1/IKKε kinases, and as an inhibitor of a hedgehog signaling pathway.
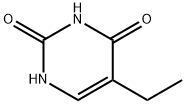
Step A: Synthesis of 2,4-dichloro-5-ethylpyrimidine. To a mixture of 5-ethyluracil (1 g, 7.1 mmol) suspended in POCl3 (4.5 mL) was slowly added N,N-dimethylaniline (1 mL). The reaction mixture was heated under reflux conditions (~120°C) for 5 hours until the feedstock was completely dissolved and the solution took on a purple color. Once the reaction was complete, the mixture was allowed to cool to room temperature and slowly poured into ice (~40 g). The resulting precipitate was filtered and washed with ice water. The precipitate was dissolved in a minimal amount of dichloromethane (DCM) and purified by passing through a short silica gel column and separated by column chromatography using DCM as eluent to afford the target product, 2,4-dichloro-5-ethylpyrimidine (1.2 g, about 100% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ8.42 (s, 1H), 2.75 (q, 2H, J=7.6 Hz), 1.29 (t, 3H, J=7.6 Hz).
[1] Patent: EP1464335, 2004, A2. Location in patent: Page/Page column 329
[2] Nucleosides and Nucleotides, 1994, vol. 13, # 1-3, p. 235 - 243
[3] Patent: WO2004/41821, 2004, A1. Location in patent: Page 26-27
[4] Synthetic Communications, 1992, vol. 22, # 20, p. 2927 - 2934
[5] Collection of Czechoslovak Chemical Communications, 2006, vol. 71, # 4, p. 579 - 594