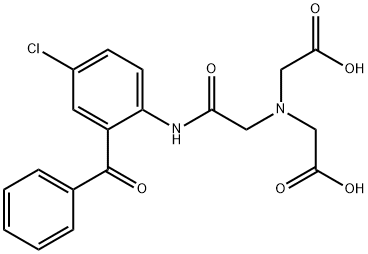
Arclofenin,ZYF Pharm Chemical
Diagnostic aid (hepatic function determination).
Arclofenin is derived from Nitrilotriacetic Acid (N490175), which is a chelating agent which forms coordination compound with metal ions. Nitrilotriacetic acid is used in complexometric titrations and as well as for protein isolation and purification in the His-tag method.
ChEBI: Arclofenin is a member of benzophenones.
A round bottom flask is charged with dimethylformamide 72.0 g, acetic
anhydride 25.0 g, pyridine 2.0 g, and nitrilotriacetic acid (NTA) 38.2 g and the
suspension is nitrogen purged for several minutes. The flask is stoppered and
the mixture is stirred at room temperature for 3 days. A small amount of
unreacted NTA is filtered out. The bulk of the solvent 79 ml is removed in
vacuo at a bath temperature of 60°-70°C. The resulting viscous solution is
twice roto-vacued after two successive additions of 40 ml dimethylformamide.
So the 2,6-diketo-N-carboxymethyl morpholine (NTA anhydride) is prepiared.
To the viscous solution of NTA anhydride is added 300 ml toluene. The
mixture is stirred at room temperature until uniform. Then 46.3 g of 2-amino-
5-chlorobenzophenone dissolved in 300 ml toluene is added to the stirred
solution of NTA anhydride and heated at 90°-100°C for 1 h. After cooling, the
reaction mixture is flashed to dryness. The residue is taken up in 400 ml of
1N sodium hydroxide and filtered. The filtrate is extracted with chloroform,
ether and charcoaled. The charcoal is removed by filtration. The product is
precipitated from the filtrate by the careful addition of 6 N HCl. The precipitate
is removed by filtration to give the N-[N'-(2-benzoyl-4-
chlorophenyl)carbamoylmethyl]iminodiacetic acid, melting point 180°-186°C
(dec., recrystallized from hot methanol/water).