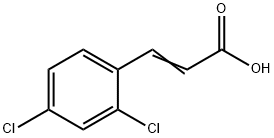
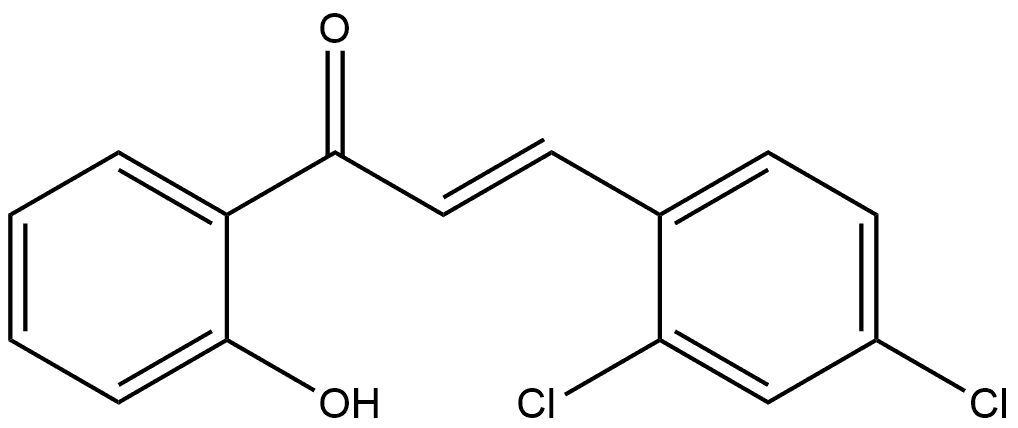
To a 5 mL round-bottomed flask equipped with a stirrer, trans-2'-hydroxychalcone (1a, 1 mmol), potassium carbonate (K2CO3, 1.38 g, 10 mmol), 35% hydrogen peroxide solution (H2O2, 0.5 mL) and acetonitrile (2 mL) were added. The reaction mixture was stirred at room temperature and the progress of the reaction was monitored by thin layer chromatography (TLC) until the complete disappearance of the ingredients. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. A 1 mol/L hydrochloric acid solution was added to the residue and the resulting solid was collected by filtration to afford the target product 2,4-dichlorostyrene acid (2a) as a white solid (133 mg, 90% yield). The structure of the product was confirmed by NMR hydrogen (1H NMR, 300 MHz, DMSO-d6) and NMR carbon (13C NMR, 300 MHz, DMSO-d6) spectra: 1H NMR (DMSO-d6) δ 7.58 (d, 1H, J = 16 Hz), 7.42-7.69 (m, 5H), 6.53 (d, 1H, J = 16 Hz); 13C NMR (DMSO-d6) δ 167.7, 144.0, 134.3, 130.3, 128.9, 128.2, 119.3.