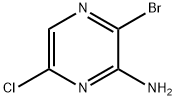
Currently, in thepreparing 2-amino-3-bromo-6-chloropyrazine, the 2-amino-6-chloropyrazine is mainly obtained by a one-step bromination reaction. According to the method, bromine is mainly positioned at an amino para position due to the activation effect of amino; generated byproducts are mainly 2-bromo-3-chloro-5-aminopyrazine and partial dibromo byproduct 2-amino-3, 5-dibromo-6-chloropyrazine, the product 2-amino-3-bromo-6-chloro is less, the yield is low. A process for preparing 2-amino-3-bromo-6-chloropyrazine uses 3-aminopyrazine-2-carboxylate as raw material and includes such steps as chlorination, diazotization bromination, ester hydrolysis, carboxyl rearrangement and removing tert-butyloxycarbonyl.