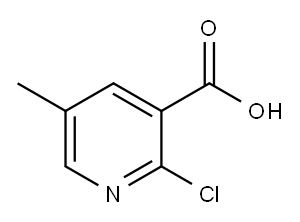
General procedure for the synthesis of 2-chloro-5-methylnicotinic acid from 2-chloro-5-methylnicotinic acid methyl ester: Intermediate 31 (2-chloro-5-methylnicotinic acid methyl ester, 0.38 g, 1.81 mmol) was dissolved in methanol (2 ml) and 2N sodium hydroxide solution (1.81 ml, 3.62 mmol) was added. The reaction mixture was stirred at room temperature for 2 hours. After completion of the reaction, the mixture was concentrated to dryness and the residue was redissolved in ethyl acetate/water. The organic layer was separated, dried over magnesium sulfate and subsequently concentrated in vacuum to afford the target product 2-chloro-5-methylnicotinic acid as a white solid in 94% yield.LRMS: m/z 172 (M + 1)+; retention time: 3.25 min.1H NMR (200 MHz, DMSO-D6) δppm: 2.2 (s, 3H); 7.6 (d, J = 2.53 Hz , 1H); 8.0 (d, J = 2.53 Hz, 1H).