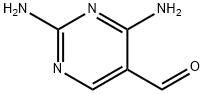
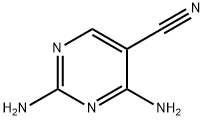
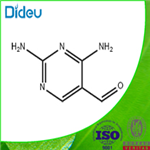
The general procedure for the synthesis of 2,4-diaminopyrimidine-5-carboxaldehyde from formic acid and 2,4-diaminopyrimidine-5-carbonitrile is as follows: nickel ruanne (2.5 g, 50% aqueous slurry) was added to a 98-100% solution of formic acid (20 mL) containing 2,4-diaminopyrimidine-5-carbonitrile (2.0 g, 14.8 mmol). The reaction mixture was refluxed for 3-4 hours. Upon completion of the reaction, the reaction mixture was filtered and the solid was washed with formic acid (10 mL). The filtrate and washings were combined and concentrated under reduced pressure. The concentrated residue was stirred in 30% aqueous ammonia solution and subsequently cooled to give a free flowing solid product. The product was collected by filtration, washed with water and dried to give 2,4-diaminopyrimidine-5-carbaldehyde (1.8 g, 78% yield) as an off-white solid.